-
Name
Diethyl cyanomethylphosphonate
- EINECS 219-806-0
- CAS No. 2537-48-6
- Article Data44
- CAS DataBase
- Density 1.122 g/cm3
- Solubility Miscible with water, chloroform, terahydrofuran, ethyl acetate and methylene chloride.
- Melting Point
- Formula C6H12NO3P
- Boiling Point 305.2 °C at 760 mmHg
- Molecular Weight 177.14
- Flash Point 117.3 °C
- Transport Information UN 1760 8/PG 2
- Appearance clear colorless to light yellow liquid
- Safety 26-28-36/37/39-23-45-27
- Risk Codes 38-36/37/38-20/21/22-34
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn,
Xn, C
C
- Synonyms Cyanomethanephosphonicacid, diethyl ester (6CI);Phosphonic acid, (cyanomethyl)-, diethyl ester(7CI,8CI,9CI);(Cyanomethyl)phosphonicacid diethyl ester;(Diethoxyphosphinyl)acetonitrile;Diethyl (cyanomethyl)phosphonate;NSC 407826;O,O-Diethyl (cyanomethyl)phosphonate;
- PSA 69.13000
- LogP 1.77608
Synthetic route

| Conditions | Yield |
|---|---|
| at 150℃; for 4h; | 99% |
| at 150℃; for 2h; | 92% |
| for 3h; Arbusov reaction; Heating; | 85% |

| Conditions | Yield |
|---|---|
| for 0.0333333h; Michaelis-Arbuzov reaction; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: acetonitrile With lithium hexamethyldisilazane In tetrahydrofuran; hexane at -78℃; for 0.5h; Metallation; Stage #2: diethyl chlorophosphate In tetrahydrofuran at -78 - 0℃; for 0.25h; Substitution; Stage #3: With hydrogenchloride In tetrahydrofuran; hexane Hydrolysis; Further stages.; | 88% |
| With lithium diisopropyl amide 1.) THF, -30 deg C, 0.5 h, 2.) -30 deg C, 5 h; Yield given. Multistep reaction; | |
| With lithium diisopropyl amide 1.) THF, -80 deg C, 15 min, 2.) THF, from -80 deg C to 0 deg C, 1 h; Multistep reaction; |
-
-
107-14-2
chloroacetonitrile

-
-
122-52-1
triethyl phosphite

-
A
-
75-00-3
chloroethane

-
B
-
2537-48-6
diethyl 1-cyanomethylphosphonate

| Conditions | Yield |
|---|---|
| at 140 - 180℃; | A n/a B 84.5% |

-
-
67-56-1
methanol

-
-
59463-50-2
diazo-cyanomethylphosphonic acid diethyl ester

-
A
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
B
-
59463-48-8
diethyl ((cyanomethoxy)methyl)phosphonate

| Conditions | Yield |
|---|---|
| In water for 3h; Irradiation; Sealed tube; Cooling with ice; | A 66% B 11% |


-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

| Conditions | Yield |
|---|---|
| With ethyl 4-nitrobenzoate In dimethyl sulfoxide at 24.9℃; Equilibrium constant; |

-
A
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
B
-
334711-70-5
2,2,2-trifluoro-N-(6-methyl-2-pyridinyl)acetamide

| Conditions | Yield |
|---|---|
| With air |
-
-
2213-63-0
2,3-dichloroquinoxaline

-
-
77679-10-8
(2-amino-2-thioxoethyl)phosphonic acid diethyl ester

-
A
-
1062508-50-2
2-chloro-3-[(3-chloro-2-quinoxalinyl)-thio]quinoxaline

-
B
-
2537-48-6
diethyl 1-cyanomethylphosphonate


| Conditions | Yield |
|---|---|
| With water; barium dihydroxide In 1,4-dioxane at 70℃; for 0.666667h; | 100% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: furfural In tetrahydrofuran at 28℃; | |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Stage #2: furfural In tetrahydrofuran; mineral oil at 0 - 25℃; for 2h; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
6836-19-7
7-Methoxy-1-tetralone

-
-
127299-26-7
(7-methoxy-3,4-dihydro-2H-naphthalene-1-ylidene)acetonitrile

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at -20 - -10℃; for 24h; Reagent/catalyst; Wittig Rearrangement; | 100% |
| Stage #1: diethyl 1-cyanomethylphosphonate With potassium hydroxide In dimethyl sulfoxide at 15 - 25℃; for 1h; Industry scale; Stage #2: 7-Methoxy-1-tetralone In tetrahydrofuran; dimethyl sulfoxide at 15 - 25℃; for 2.5h; Product distribution / selectivity; | 95% |
| Stage #1: 7-Methoxy-1-tetralone With sodium hydride In 1,2-dimethoxyethane at 15℃; for 0.5h; Stage #2: diethyl 1-cyanomethylphosphonate In monoethylene glycol diethyl ether at 10 - 15℃; | 89% |

| Conditions | Yield |
|---|---|
| With caesium carbonate at 20℃; for 24h; Horner reaction; | 100% |
| With sodium hydride 1.) THF, RT, 20 min, 2.) THF, RT, 72 h; Multistep reaction; | |
| In tetrahydrofuran at 0℃; for 3h; Yield given; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at -70℃; for 0.666667h; Inert atmosphere; | 100% |
| With lithium hexamethyldisilazane In tetrahydrofuran at -70℃; for 0.666667h; Inert atmosphere; | 100% |
| With lithium bromide In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
219130-11-7
(4E,6E)-5-Methyl-7-(2,6,6-trimethyl-cyclohex-1-enyl)-hepta-4,6-dienal

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
66-77-3
1-naphthaldehyde

-
-
111686-30-7
3-(1-naphthyl)acrylonitrile

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 20℃; Wadsworth-Emmons reaction; | 100% |
| With potassium carbonate for 0.25h; Ambient temperature; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
345-70-0
3,3'-difluorobenzophenone

-
-
170019-08-6
3,3'-bis(3-fluorophenyl)acrylonitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide | 100% |
| With sodium hydride In N,N-dimethyl-formamide Horner-Emmons coupling; | 100% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.416667h; Stage #2: 3,3'-difluorobenzophenone In tetrahydrofuran; mineral oil at 80℃; Cooling with ice; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
14674-38-5
N-(4-methoxybenzylidene)-p-toluenesulfonamide

-
-
36315-64-7
diethyl [(E)-1-cyano-2-(4-methoxyphenyl)ethenyl]phosphonate

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide at 20℃; for 1h; Knoevenagel condensation; Inert atmosphere; Molecular sieve; optical yield given as %de; stereoselective reaction; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 0.0333333h; Condensation; | 81% |
| Stage #1: diethyl 1-cyanomethylphosphonate With potassium tert-butylate In dimethyl sulfoxide Knoevenagel type condensation; Stage #2: N-(4-methoxybenzylidene)-p-toluenesulfonamide In dimethyl sulfoxide at 20℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; for 1.5h; | 100% |
-
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
54354-52-8
3,5-Dimethyl-2,4-hexadienenitrile

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -60 - 20℃; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
153507-02-9
3-(3,5-dimethoxyphenyl)acrylonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 20℃; for 0.25h; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at 20℃; for 17h; | 100% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 3,5-dimethoxybenzaldehdye In tetrahydrofuran at 28℃; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
1187594-26-8
1-[(1-methylcyclopropyl)sulfonyl]azetidin-3-one

-
-
1187594-27-9
2-(1-((1-methylcyclopropyl)sulfonyl)azetidin-3-ylidene)acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; Stage #2: 1-[(1-methylcyclopropyl)sulfonyl]azetidin-3-one In tetrahydrofuran; mineral oil at 20℃; for 16h; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
1236306-10-7
2-(5,5-dichloro-6,7-dihydro-1-tosyl-1H-indol-4(5H)-ylidene)acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.5h; Inert atmosphere; Stage #2: 5,5-dichloro-4-oxo-1-(p-toluenesulfonyl)-4,5,6,7,-tetrahydroindole In tetrahydrofuran; mineral oil at 0 - 20℃; Inert atmosphere; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
59379-02-1
tert-butyl 3-formylpyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 2h; Stage #2: tert-butyl 3-formylpyrrolidine-1-carboxylate In tetrahydrofuran at 0 - 20℃; for 16h; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 12h; | 86.1% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
5623-81-4
2-cyclopentylacetaldehyde

-
-
1269823-36-0
4-cyclopentylbut-2-enenitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 1h; Stage #2: 2-cyclopentylacetaldehyde In tetrahydrofuran; mineral oil at 20℃; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
103893-16-9
4-N,N-di-n-butylamino-2-methoxybenzaldehyde

-
-
1267604-90-9
3-(4-dibutylamino-2-methoxyphenyl)acrylonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran for 0.416667h; Cooling with ice; Stage #2: 4-N,N-di-n-butylamino-2-methoxybenzaldehyde In tetrahydrofuran for 1h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With potassium tert-butylate In tetrahydrofuran at 5 - 20℃; for 1h; Inert atmosphere; Stage #2: C12H16N2O In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

| Conditions | Yield |
|---|---|
| With lithium bromide at 100℃; for 70h; | 100% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
1208005-62-2
7-diethylamino-9,9-dimethylfluorene-2-carbaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 7-diethylamino-9,9-dimethylfluorene-2-carbaldehyde In tetrahydrofuran at 20℃; for 4h; | 100% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.5h; Stage #2: 7-diethylamino-9,9-dimethylfluorene-2-carbaldehyde In tetrahydrofuran; mineral oil at 20℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 1h; Stage #2: C19H34O4Si In tetrahydrofuran; hexane at 0 - 20℃; Wittig-Horner Reaction; | 100% |
-
-
4746-97-8
cyclohexanedione monoethylene ketal

-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
124499-35-0
2-<4,4-(ethylenedioxy)cyclohexylidene>acetonitrile

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; sodium hydride In tetrahydrofuran; paraffin for 12h; | 99% |
| Stage #1: diethyl 1-cyanomethylphosphonate With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; sodium hydride In tetrahydrofuran for 0.416667h; Inert atmosphere; Stage #2: cyclohexanedione monoethylene ketal at 20℃; for 3h; | 96% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran Stage #2: cyclohexanedione monoethylene ketal With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone In tetrahydrofuran | 95% |
-
-
64872-91-9
2-(1,3-dithian-2-yl)-2-methylpropanal

-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
139745-78-1
(E)-4-(1,3-dithian-2-yl)-4-methylpent-2-enenitrile

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran | 99% |
| With lithium diisopropyl amide In tetrahydrofuran; hexane 1.) -78 deg C, 45 min; 2.) -78 deg C, 2 h; RT, 2 h; | 99% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
144617-64-1
6,6'-dideuterio-3,3'-dimethylbenzophenone

-
-
144617-69-6
C17H13(2)H2N

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,2-dimethoxyethane | 99% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
120-92-3
cyclopentanone

-
-
5732-88-7
cyclopentylideneacetonitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In diethyl ether at 20℃; Cooling with ice; | 99% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In N,N-dimethyl-formamide at 0℃; Stage #2: cyclopentanone In N,N-dimethyl-formamide at 0 - 20℃; | 89% |
| With potassium carbonate In water at 80℃; for 2.5h; | 88% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
19637-35-5
3-tetrahydropyran-2-yloxy-androst-5-en-17-one

| Conditions | Yield |
|---|---|
| With sodium hydride Wittig-Horner reaction; | 99% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In 1,2-dimethoxyethane for 0.166667h; Heating; Stage #2: 3-tetrahydropyran-2-yloxy-androst-5-en-17-one In 1,2-dimethoxyethane for 5h; Wittig-Horner reaction; Heating; Further stages.; | 99% |
-
-
5453-80-5, 19926-90-0
5-Norbornene-2-carboxaldehyde

-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

| Conditions | Yield |
|---|---|
| With polymer-anchored quaternary trimethylammonium hydroxide In acetonitrile at 20℃; for 1h; Horner-Emmons olefination; | 99% |
-
-
398489-26-4
tert-butyl 3-oxoazetidine-1-carboxylate

-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
1153949-11-1
3-(cyanomethylene)azetidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; Stage #2: tert-butyl 3-oxoazetidine-1-carboxylate In tetrahydrofuran; mineral oil Product distribution / selectivity; | 99% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 0 - 20℃; Stage #2: tert-butyl 3-oxoazetidine-1-carboxylate In tetrahydrofuran at 0℃; Stage #3: With water In tetrahydrofuran | 98% |
| Stage #1: diethyl 1-cyanomethylphosphonate With potassium tert-butylate In tetrahydrofuran at 0 - 30℃; Stage #2: tert-butyl 3-oxoazetidine-1-carboxylate In tetrahydrofuran at 0 - 30℃; | 95% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
30830-27-4
3-benzyloxy cyclobutanone

-
-
1187595-82-9
2-(3-(benzyloxy)cyclobutylidene)acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With triethylamine; lithium bromide In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 3-benzyloxy cyclobutanone at 20℃; for 20h; Concentration; | 99% |
| Stage #1: diethyl 1-cyanomethylphosphonate With triethylamine; lithium bromide In tetrahydrofuran at 10℃; for 1.5h; Inert atmosphere; Stage #2: 3-benzyloxy cyclobutanone In tetrahydrofuran at 10℃; for 20h; Inert atmosphere; | 91% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: 3-benzyloxy cyclobutanone In tetrahydrofuran at 20℃; | 80% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: 3-benzyloxy cyclobutanone In tetrahydrofuran at 20℃; Inert atmosphere; | 80% |
| Stage #1: diethyl 1-cyanomethylphosphonate With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; Stage #2: 3-benzyloxy cyclobutanone In tetrahydrofuran at 0 - 20℃; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
123-08-0
4-hydroxy-benzaldehyde

-
-
1694-92-4
2-Nitrobenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy-benzaldehyde; 2-Nitrobenzenesulfonyl chloride With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 1.5h; Stage #2: diethyl 1-cyanomethylphosphonate With lithium chloride at 20℃; for 24h; Inert atmosphere; | 99% |

Diethyl cyanomethylphosphonate Chemical Properties
IUPAC Name: 2-diethoxyphosphorylacetonitrile
Empirical Formula: C6H12NO3P
Molecular Weight: 177.1381
EINECS: 219-806-0
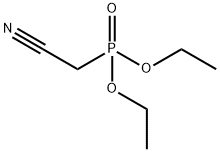
Structure of Phosphonicacid, P-(cyanomethyl)-, diethyl ester (CAS NO.2537-48-6):
Index of Refraction: 1.422
Molar Refractivity: 40.12 cm3
Molar Volume: 157.8 cm3
Polarizability: 15.9×10-24cm3
Surface Tension: 37.5 dyne/cm
Density: 1.122 g/cm3
Flash Point: 117.3 °C
Enthalpy of Vaporization: 54.57 kJ/mol
Boiling Point: 305.2 °C at 760 mmHg
Vapour Pressure: 0.000831 mmHg at 25°C
Product Categories: Phosphorus compounds;Horner-Emmons Reaction;Synthetic Organic Chemistry;Wittig & Horner-Emmons Reaction
Diethyl cyanomethylphosphonate Safety Profile
Hazard Codes:  Xi,
Xi, Xn,
Xn, C
C
Risk Statements: 38-36/37/38-20/21/22-34
R38:Irritating to skin.
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R34:Causes burns.
Safety Statements: 26-28-36/37/39-23-45-27
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S23:Do not breathe vapour.
S25:Avoid contact with eyes.
S27:Take off immediately all contaminated clothing.
RIDADR: UN 1760 8/PG 2
WGK Germany: 3
Hazard Note: Irritant
HazardClass: 6.1
PackingGroup: III
HS Code: 29310095
Diethyl cyanomethylphosphonate Specification
Phosphonicacid, P-(cyanomethyl)-, diethyl ester , its cas register number is 2537-48-6. It also can be called (Cyanomethyl)diethoxyphosphine oxide ; Diethyl (cyanomethyl)phosphonate ; Diethyl cyanomethanephosphonate ; Diethyl-(cyanmethyl)phosphonat ; Phosphonic acid, (cyanomethyl)-, diethyl ester ; (Diethylphosphono)acetonitrile ; 2-(Diethylphosphonyl)acetonitrile . Phosphonicacid, P-(cyanomethyl)-, diethyl ester (CAS NO.2537-48-6) is a clear colorless to light yellow liquid.
Related Products
- Diethyl (1-phenylpropyl)malonate
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(diethoxymethylsilyl)ethyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (beta,gamma-epoxypropyl)phosphonate
- 2537-62-4
- 253764-88-4
- 253771-21-0
- 25377-72-4
- 25377-73-5
- 25377-91-7
- 253787-45-0
- 25379-20-8
- 253801-04-6
- 253-82-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View