 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
-
Name
Diethyl ether
- EINECS 200-467-2
- CAS No. 60-29-7
- Article Data681
- CAS DataBase
- Density 0.734 g/cm3
- Solubility 69 g/L (20 °C) in water
- Melting Point -116 °C
- Formula C4H10O
- Boiling Point 33.177 °C at 760 mmHg
- Molecular Weight 74.1228
- Flash Point -45 °C
- Transport Information UN 1155 3/PG 1
- Appearance Colourless liquid
- Safety 9-16-29-33-45-36/37
- Risk Codes 12-19-22-66-67-39/23/24/25-23/24/25
-
Molecular Structure
-
Hazard Symbols
 F+,
F+,  Xn,
Xn,  T
T
- Synonyms Ether(6CI);Ethyl ether (8CI);1,1'-Oxybisethane;3-Oxapentane;Anaesthetic ether;Anesthesia ether;Anesthetic ether;Diethyl oxide;Ethoxyethane;Ethyl oxide;NSC 100036;Pronarcol;Sulfuric ether;Ethyl ether;Ethane, 1,1'-oxybis-;
- PSA 9.23000
- LogP 1.04280
Synthetic route

| Conditions | Yield |
|---|---|
| With reduced Sn/hydrotalcite catalyst at 250℃; under 25502.6 Torr; Catalytic behavior; Reagent/catalyst; Temperature; Pressure; | 99% |
| With SA5 at 199.84℃; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; | 2.6% |
| With sulfuric acid at 130 - 140℃; Darstellung im grossen; |

| Conditions | Yield |
|---|---|
| With alumina at 449.84℃; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; Overall yield = 100 %; | A 0.1% B 98.9% |
| C2I2O2Rh(1-)*C8H20N(1+); tetraethylammonium iodide; hydrogen iodide In water at 110℃; Product distribution / selectivity; Inert atmosphere; Autoclave; | A 10% B 50% |
| 1-methyl-3-(propyl-3-sulfonyl)imidazolium trifluoromethanesulfonate; CF3O3S(1-)*CHF3O3S*C7H13N2O3S(1+) at 240 - 260℃; for 4h; Product distribution / selectivity; | A n/a B 12% |
-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
-
65039-09-0
1-ethyl-3-methyl-1H-imidazol-3-ium chloride

-
A
-
377739-43-0
1-ethyl-3-methyl-imidazolium tris(pentafluoroethyl)trifluorophosphate

-
B
-
60-29-7
diethyl ether

-
C
-
75-00-3
chloroethane

| Conditions | Yield |
|---|---|
| at 80℃; for 3h; Product distribution / selectivity; | A 98.9% B n/a C n/a |

-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
-
30388-20-6
N,N,N',N',N'',N''-hexamethylguanidinium chloride

-
B
-
60-29-7
diethyl ether

-
C
-
75-00-3
chloroethane

| Conditions | Yield |
|---|---|
| at 80℃; for 3h; | A 98.9% B n/a C n/a |

-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
-
155371-19-0
1-ethyl-3-methylimidazolium hexafluorophosphate

-
A
-
377739-43-0
1-ethyl-3-methyl-imidazolium tris(pentafluoroethyl)trifluorophosphate

-
B
-
60-29-7
diethyl ether

-
C
-
353-36-6
1-fluoroethane

-
D
-
7647-19-0, 874483-74-6
phosphorus pentafluoride

| Conditions | Yield |
|---|---|
| at 100℃; for 10h; Product distribution / selectivity; | A 98.7% B n/a C n/a D n/a |

-
-
945614-34-6
triethyloxonium bis(trifluoromethylsulfonyl)imide

-
-
59016-54-5
1-cyano-4-N,N-dimethylaminopyridinium bromide

-
A
-
74-96-4
ethyl bromide

-
B
-
60-29-7
diethyl ether

-
C
-
945614-38-0
1-cyano-4-dimethylaminopyridinium bis(trifluoromethylsulfonyl)imide

| Conditions | Yield |
|---|---|
| at 60℃; for 5h; | A n/a B n/a C 98.2% |

-
-
945614-34-6
triethyloxonium bis(trifluoromethylsulfonyl)imide

-
-
65039-09-0
1-ethyl-3-methyl-1H-imidazol-3-ium chloride

-
A
-
60-29-7
diethyl ether

-
B
-
75-00-3
chloroethane

-
C
-
174899-82-2
1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide

| Conditions | Yield |
|---|---|
| at 80℃; for 3h; | A n/a B n/a C 97.9% |

-
-
766-77-8
Dimethylphenylsilane

-
-
123-63-7
paracetaldehyde

-
A
-
60-29-7
diethyl ether

-
B
-
56-33-7
1,1,3,3-tetramethyl-1,3-diphenyldisiloxane

| Conditions | Yield |
|---|---|
| With (pentamethylcyclopentadienyl)Ge(II)+B(ArF)4- In dichloromethane-d2 at 50℃; Catalytic behavior; Reagent/catalyst; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With triethylsilane; [CpW(CO)2(1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene)]B(C6F5)4 at 23℃; for 26h; Conversion of starting material; | A 5.9% B 96.3% |

-
-
3790-23-6
tetraethyldiphosphine disulfide

-
A
-
115018-02-5
((CH3)5C5)2Yb(S2P(C2H5)2)

-
B
-
60-29-7
diethyl ether

-
C
-
3040-63-9
1,1,2,2-tetraethyldiphosphane

| Conditions | Yield |
|---|---|
| In toluene stirring, 2 h, under N2; concn., cooling to -10°C; elem. anal.; | A 96% B n/a C n/a D n/a |

-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
A
-
60-29-7
diethyl ether

-
B
-
75-00-3
chloroethane

-
C
-
916807-26-6
1-decyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate

| Conditions | Yield |
|---|---|
| at 80℃; for 3h; | A n/a B n/a C 96% |

-
-
776-76-1
methyldiphenylsilane

-
-
123-63-7
paracetaldehyde

-
A
-
60-29-7
diethyl ether

-
B
-
807-28-3
1,3-Dimethyl-1,1,3,3-tetraphenyldisiloxan

| Conditions | Yield |
|---|---|
| With (pentamethylcyclopentadienyl)Ge(II)+B(ArF)4- In dichloromethane-d2 at 50℃; Catalytic behavior; | A n/a B 96% |

-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
-
76-83-5
trityl chloride

-
A
-
60-29-7
diethyl ether

-
B
-
75-00-3
chloroethane

| Conditions | Yield |
|---|---|
| at 80℃; for 10h; | A n/a B n/a C 93.6% |

-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
-
59016-54-5
1-cyano-4-N,N-dimethylaminopyridinium bromide

-
A
-
74-96-4
ethyl bromide

-
B
-
60-29-7
diethyl ether

-
C
-
945614-37-9
1-cyano-4-dimethylaminopyridinium tris(pentafluoroethyl)trifluorophosphate

| Conditions | Yield |
|---|---|
| at 60℃; for 5h; | A n/a B n/a C 93.2% |


-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
A
-
60-29-7
diethyl ether

-
B
-
75-00-3
chloroethane

-
C
-
945614-40-4
1-hexyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate

| Conditions | Yield |
|---|---|
| at 80℃; for 3h; | A n/a B n/a C 93% |


| Conditions | Yield |
|---|---|
| With NaX faujasite at 180 - 240℃; for 6h; | A 93% B 5% |
-
-
74-85-1
ethene

-
-
64-19-7
acetic acid

-
A
-
60-29-7
diethyl ether

-
B
-
64-17-5
ethanol

-
C
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: acetic acid With water at 92.4℃; Stage #2: ethene; cesium nitrate; tungstophosphoric acid; water; mixture of, dried, tabletted at 92.4 - 194.4℃; under 6750.68 Torr; Product distribution / selectivity; Gas phase; | A 3.2% B 3.6% C 92.7% |
| With water; cesium nitrate; tungstophosphoric acid; water; mixture of, dried, tabletted at 92.4 - 165℃; under 6750.68 Torr; Product distribution / selectivity; Gas phase; | A 3% B 3.4% C 91.5% |
| With water; lithium nitrate; silica; tungstophosphoric acid; water; mixture of, heated at 150 C at 102.2 - 165℃; under 6750.68 Torr; Product distribution / selectivity; Gas phase; | A 2.2% B 5% C 90.1% |
| With water; lithium nitrate; silica; tungstosilicic acid; water; mixture of, heated at 150 C at 102.2 - 165℃; under 6750.68 Torr; Conversion of starting material; Gas phase; | A 4.7% B 7.6% C 87.7% |

-
-
74-85-1
ethene

-
-
79-10-7
acrylic acid

-
A
-
60-29-7
diethyl ether

-
B
-
64-17-5
ethanol

-
C
-
140-88-5
ethyl acrylate

| Conditions | Yield |
|---|---|
| With water; cesium nitrate; tungstophosphoric acid; water; mixture of, dried, tabletted at 85.6 - 165℃; under 2250.23 Torr; Product distribution / selectivity; Gas phase; | A 3.5% B 4.3% C 91.8% |

-
-
64-17-5
ethanol

-
-
15148-19-3
1,3-bis(p-nitrophenyl)-2-thia-1,3-diazaallene

-
A
-
60-29-7
diethyl ether

-
B
-
623-81-4
diethyl sulphite

-
C
-
100-01-6
4-nitro-aniline

| Conditions | Yield |
|---|---|
| With copper dichloride for 24h; Product distribution; Ambient temperature; other reagent; | A 93.6 % Chromat. B 70% C 91% |

-
-
945614-32-4
triethyloxonium tris(pentafluoroethyl)trifluorophosphate

-
-
143314-16-3
1-ethyl-3-methylimidazolium tetrafluoroborate

-
A
-
377739-43-0
1-ethyl-3-methyl-imidazolium tris(pentafluoroethyl)trifluorophosphate

-
B
-
60-29-7
diethyl ether

-
C
-
353-36-6
1-fluoroethane

-
D
-
7637-07-2
boron trifluoride

| Conditions | Yield |
|---|---|
| at 100℃; for 10h; Product distribution / selectivity; | A 90.3% B n/a C n/a D n/a |

-
-
60-29-7
diethyl ether

-
-
74415-68-2
hexafluoro-3-oxatricyclo<3.2.0.02,4>hept-6-ene

-
A
-
353-36-6
1-fluoroethane

| Conditions | Yield |
|---|---|
| for 2160h; Ambient temperature; | A 100% B 75% |
| for 2160h; Yields of byproduct given; | A n/a B 21% |


| Conditions | Yield |
|---|---|
| With hexafluoro-3-oxatricyclo<3.2.0.02,4>hept-6-ene for 2160h; Ambient temperature; | A 100% B 75% |
-
-
60-29-7
diethyl ether

-
-
68602-57-3
trifluoroacetyl triflate

-
A
-
383-63-1
ethyl trifluoroacetate,

-
B
-
425-75-2
trifluoromethanesulfonic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 0℃; | A 100% B 100% |

-
-
60-29-7
diethyl ether

| Conditions | Yield |
|---|---|
| With lithium at 20℃; for 20h; Reduction; | 100% |
-
-
60-29-7
diethyl ether


| Conditions | Yield |
|---|---|
| With lithium at 20℃; for 20h; Reduction; | 100% |
-
-
60-29-7
diethyl ether

-
-
444-30-4
2-(trifluoromethyl)phenol

-
-
35335-16-1
1,4-bis(bromomethyl)-2,5-dibromobenzene

-
-
474330-25-1
1,4-dibromo-2,5-bis(2-trifluoromethylphenoxymethyl)benzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium carbonate In dichloromethane; acetone | 100% |

-
-
193902-67-9
4-[[[4-[4-(ethyloxycarbonyl)-1-piperazinyl]phenyl]amino]carbonyl]-1-t-butyloxycarbonyl-piperidine

-
-
60-29-7
diethyl ether

-
-
193902-68-0
4-[[[4-[4-(ethyloxycarbonyl)-1-piperazinyl]phenyl]amino]carbonyl]piperidine

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; hydrogenchloride | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 3,4-dihydro-2H-pyran | 100% |
-
-
79-37-8
oxalyl dichloride

-
-
60-29-7
diethyl ether

-
-
20017-67-8
3,3-diphenylpropan-1-ol

-
-
4279-81-6
3,3-diphenylpropanal

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide; triethylamine In dichloromethane | 100% |

-
-
63606-55-3
2-(2-ethyl-benzofuran-3-yl)-propionic acid

-
-
60-29-7
diethyl ether

-
-
63606-56-4
2-(2-ethyl-benzofuran-3-yl)-propionamide

| Conditions | Yield |
|---|---|
| In thionyl chloride | 100% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane | 100% |
| With n-butyllithium In hexane |
-
-
60-29-7
diethyl ether

-
-
52341-13-6, 12264-20-9
trans-dichloro(ethylene)(2,4,6-trimethylpyridine)platinum

-
-
91068-18-7
trans-dichloro(diethyl ether)(2,4,6-trimethylpyridine)platinum(II)

| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: ethylene; Irradiation (UV/VIS); | 100% |
| In diethyl ether Irradiation (UV/VIS); the Pt-complex dissolved in Et2O was introduced into a muffshaped Schlenk tube surrounding a 125-W medium-pressure mercury lamp, Philips HPK 125, irradn. for 15 min at room temp., λ<310 nm was eliminated by Pyrex filter; the solvent was removed under reduced pressure at -30°C, the solid was recrystd. at -30°C in pentane-CH2Cl2; | 95% |

| Conditions | Yield |
|---|---|
| In diethyl ether (high vac. line); condensing gallium complex in an ampoule with Et2O, warming to room temp. over a period of 30 min; fractionation, collection in a trap at -30°C; | 100% |
-
-
60-29-7
diethyl ether

-
-
293764-40-6
[(C5H4N)C(CH3)(CH2N(C6H2(CH3)3))2]Zr(CH3)2

-
-
917-54-4
methyllithium

-
-
486413-21-2
[C5H4NC(CH3)(CH2NC6H2(CH3)3)2]Zr(methyl)3[Li*diethyl ether]

| Conditions | Yield |
|---|---|
| In diethyl ether N2; addn. of methyllithium as 4.4 M ether soln. to ether suspn. of Zr complex at -30° C, stirring at room temp. for 10 min; filtration through Celite, drying the filtrate in vac.; elem. anal.; | 100% |


| Conditions | Yield |
|---|---|
| In diethyl ether; benzene-d6 byproducts: ethane; N2-atmosphere; room temp. (20 min); | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In benzene-d6 byproducts: ethane; N2-atmosphere; room temp. (10 min); evapn. (vac.); | 100% |
-
-
60-29-7
diethyl ether

| Conditions | Yield |
|---|---|
| In dichloromethane (inert conditions); removal of volatiles (vac.); | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether (N2), GaCl3 in Et2O added dropwise to soln. of LiAlH4 in Et2O at 0°C, stirred at 0°C for 2 h; stored overnight at -20°C, filtered cold, evapd. at -78°C; | 100% |

-
-
60-29-7
diethyl ether

-
A
-
1244773-04-3
(1S,3aR,5S,5'S,6R,6a'R)-2,2'-dimethyl-5'-(2-methyl-1,3-dioxolan-4-yl)dihydro-3a'H-3-oxaspiro[bicyclo[3.2.0.]heptane-6,6'-furo[2,3-d][1,3]dioxole]

-
B
-
108-94-1
cyclohexanone

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); benzene Inert atmosphere; Irradiation; | A 65% B 100% |

-
-
60-29-7
diethyl ether

-
-
1235436-62-0
bis(trimethylsilyl)-trifluoromethylsulfonium tetrakis(pentafluorophenyl)borate

| Conditions | Yield |
|---|---|
| react. bis(trimethylsilyl)-trifluoromethylsulfonium tetrakis(pentafluorophenyl)borate with Et2O; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 12h; | 100% |


| Conditions | Yield |
|---|---|
| at 20℃; for 12h; | 100% |


| Conditions | Yield |
|---|---|
| at 20℃; for 1h; Inert atmosphere; | 100% |

-
-
60-29-7
diethyl ether

-
-
1445605-48-0
Ce(decafluorodiphenylamide)3(diethyl ether)2

| Conditions | Yield |
|---|---|
| for 0.5h; Inert atmosphere; | 100% |

-
-
60-29-7
diethyl ether

-
-
1365891-80-0, 1365891-81-1
(R,Rb)-[1,1'-binaphthalene]-2,2'-diyl(2'-methoxy-[1,1'-binaphthalen]-2-yl)phosphonite

-
-
1436385-41-9
[RhCl((R,R)-C41H27O3P)(η4-cod)]*(C2H5)2O

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; Inert atmosphere; Schlenk technique; | 100% |


| Conditions | Yield |
|---|---|
| In toluene Inert atmosphere; Glovebox; | 100% |


| Conditions | Yield |
|---|---|
| With alumina at 270℃; under 760.051 Torr; Inert atmosphere; Gas phase; Green chemistry; | 100% |

| Conditions | Yield |
|---|---|
| With magnesium Heating; | 100% |
Diethyl ether History
Alchemist Raymundus Lullus is credited with discovering the compound in 1275 AD, although there is no contemporary evidence of this.
Ethyl ether (CAS NO.60-29-7) was first synthesized in 1540 by Valerius Cordus, who called it "oil of sweet vitriol" (oleum dulcis vitrioli)—the name was because it was originally discovered by distilling a mixture of ethanol and sulfuric acid (then known as oil of vitriol)—and noted some of its medicinal properties. At about the same time, Theophrastus Bombastus von Hohenheim, better known as Paracelsus, discovered ether's analgesic properties. In 1730 ,August Siegmund Frobenius gave the name ether to the substance .
Diethyl ether Consensus Reports
IARC Cancer Review: Animal No Adequate Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 , 1987,p. 93.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Diethyl ether Standards and Recommendations
OSHA PEL: TWA 400 ppm; STEL 500 ppm
ACGIH TLV: TWA 400 ppm; STEL 500 ppm
DFG MAK: 400 ppm (1200 mg/m3)
DOT Classification: 3; Label: Flammable Liquid
Diethyl ether Analytical Methods
For occupational chemical analysis use NIOSH: Ethyl Ether, 1610.
Diethyl ether Specification
The Ethyl ether with CAS registry number of 60-29-7 is also known as Diethyl ether. The IUPAC name is Ethoxyethane. It belongs to product categories of Refrigerants; Anhydrous Solvents; Synthetic Organic Chemistry; Chemistry. Its EINECS registry number is 200-467-2. In addition, the formula is C4H10O and the molecular weight is 74.12. This chemical is a colourless liquid and should be cool and dry place away from oxidizing agents.
Physical properties about Ethyl ether are: (1)ACD/LogP: 1.04; (2)ACD/LogD (pH 5.5): 1.041; (3)ACD/LogD (pH 7.4): 1.041; (4)ACD/BCF (pH 5.5): 3.638; (5)ACD/BCF (pH 7.4): 3.638; (6)ACD/KOC (pH 5.5): 87.722; (7)ACD/KOC (pH 7.4): 87.722; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 2; (10)Polar Surface Area: 9.23Å2; (11)Index of Refraction: 1.361; (12)Molar Refractivity: 22.326 cm3; (13)Molar Volume: 100.925 cm3; (14)Surface Tension: 19.09 dyne/cm; (15)Density: 0.734 g/cm3; (16)Enthalpy of Vaporization: 26.52 kJ/mol; (17)Boiling Point: 33.177 °C at 760 mmHg; (18)Vapour Pressure: 566.752 mmHg at 25 °C.
Preparation of Ethyl ether. Firstly, mix ethanol (95%) and concentrated sulfuric acid (98%) with ratio of 1:1 and heat the reaction mixture to 83 °C. Then pass into ethanol vapor and stir the mixture at 120-125 °C. After the reaction, gaseous product is washed through sodium hydroxide solution, saturated solution of sodium bisulfite and distilled water at atmospheric pressure. At last, product is obtained by collecting fraction of 33.5-34.5 °C.
![]()
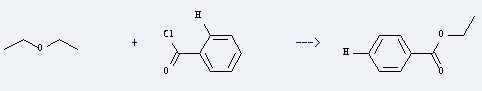
Uses of Ethyl ether: it is important anesthetic in medicine. Besides, it is used as analytical reagent for nickel, potassium, magnesium and other analysis. What's more, it is used as solvent, cleaning agent and also used in medicine, electronic and other industries. For example, it is used to produce benzoic acid ethyl ester by reaction with benzoyl chloride. The reaction needs catalyst anhydrous cobalt(II) chloride and solvent acetonitrile at ambient temperature for 8 hours. The tield is about 49%.
When you are using this chemical, please be cautious about it. As a chemical, it is extremely flammable and may form explosive peroxides. What's more, it has danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. Besides, its repeated exposure may cause skin dryness or cracking vapours may cause drowsiness and dizziness. During using it, wear suitable protective clothing and gloves and do not empty into drains. Keep container in a well-ventilated place and away from sources of ignition. In case of accident or if you feel unwell seek medical advice immediately. It is necessary to take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCOCC
2. InChI: InChI=1S/C4H10O/c1-3-5-4-2/h3-4H2,1-2H3
3. InChIKey: RTZKZFJDLAIYFH-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LCLo | inhalation | 76000ppm (76000ppm) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1294, 1935. | |
| frog | LDLo | subcutaneous | 24gm/kg (24000mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1295, 1935. | |
| guinea pig | LDLo | intraperitoneal | 2gm/kg (2000mg/kg) | American Industrial Hygiene Association Journal. Vol. 35, Pg. 21, 1974. | |
| human | TCLo | inhalation | 200ppm (200ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 282, 1943. |
| man | LDLo | oral | 260mg/kg (260mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| mouse | LC50 | inhalation | 31000ppm/30M (31000ppm) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Experimental Animals. Jikken Dobutso Iho. Vol. 34, Pg. 379, 1985. |
| mouse | LD50 | intraperitoneal | 2420mg/kg (2420mg/kg) | Proceedings of the Western Pharmacology Society. Vol. 27, Pg. 511, 1984. | |
| mouse | LD50 | intravenous | 996mg/kg (996mg/kg) | Journal of Pharmaceutical Sciences. Vol. 67, Pg. 566, 1978. | |
| mouse | LDLo | subcutaneous | 8mg/kg (8mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1295, 1935. | |
| rabbit | LCLo | inhalation | 106000ppm (106000ppm) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1294, 1935. | |
| rabbit | LD50 | skin | > 20mL/kg (20mL/kg) | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. | |
| rat | LCLo | inhalation | 32000ppm/4H (32000ppm) | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. | |
| rat | LD50 | oral | 1215mg/kg (1215mg/kg) | Toxicology and Applied Pharmacology. Vol. 19, Pg. 699, 1971. |
Related Products
- Diethyl (1-phenylpropyl)malonate
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(diethoxymethylsilyl)ethyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (beta,gamma-epoxypropyl)phosphonate
- 60299-11-8
- 603-00-9
- 6030-36-0
- 60303-68-6
- 60304-36-1
- 60308-49-8
- 603107-76-2
- 60-31-1
- 60311-02-6
- 603-11-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View