-
Name
DIETHYL ETHYLPHOSPHONATE
- EINECS 201-111-9
- CAS No. 78-38-6
- Article Data141
- CAS DataBase
- Density 1.01 g/cm3
- Solubility Miscible with water or organic solvents
- Melting Point
- Formula C6H15O3P
- Boiling Point 198 °C at 760 mmHg
- Molecular Weight 166.157
- Flash Point 92.7 °C
- Transport Information UN 3082 9/PG 3
- Appearance Colorless liquid with a mild odor
- Safety 37/39-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Ethanephosphonic acid, diethyl ester;Phosphonic acid, ethyl-, diethyl ester;CCRIS 6230;AI3-18558;HSDB 2560;NSC 2671;1-[Ethoxy(ethyl)phosphoryl]oxyethane;
- PSA 45.34000
- LogP 2.27240
Synthetic route

| Conditions | Yield |
|---|---|
| at 180 - 200℃; for 0.416667h; Michaelis-Arbuzov reaction; microwave irradiation; | 100% |
| at 100℃; for 12h; reaction de Michaelis Arbuzov'a; | 95% |
| for 24h; Michaelis-Arbuzov Synthesis; Reflux; | 80% |
| In neat (no solvent) at 150℃; for 0.833333h; Temperature; Arbuzov Reaction; Flow reactor; | 5% |
| at 150℃; for 4h; Arbuzov Reaction; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 150℃; for 0.833333h; Temperature; Arbuzov Reaction; Flow reactor; | 99% |
| for 3h; Heating; | 98% |
| for 3h; Reflux; | 98% |
-
-
1765-40-8
(bromomethyl)pentafluorobenzene

-
-
122-52-1
triethyl phosphite

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| at 160℃; for 12h; | A n/a B 99% |

-
-
100-39-0
benzyl bromide

-
-
122-52-1
triethyl phosphite

-
A
-
74-96-4
ethyl bromide

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

-
C
-
1080-32-6
O,O-diethyl benzylphosphonate

| Conditions | Yield |
|---|---|
| at 140℃; for 0.25h; Michaelis-Arbuzov reaction; microwave irradiation; | A n/a B n/a C 98% |


| Conditions | Yield |
|---|---|
| at 90 - 170℃; for 0.166667h; Arbuzov Reaction; | A n/a B 98% C n/a |

-
-
122-52-1
triethyl phosphite

-
A
-
64-67-5
diethyl sulfate

-
B
-
78-40-0
triethyl phosphate

-
C
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sulfur trioxide In dichloromethane at -78℃; | A 97% B 2% C 1% |
| With sulfur trioxide In dichloromethane at -78℃; | A 17% B 55% C 28% |


| Conditions | Yield |
|---|---|
| With aluminum oxide at 20℃; | 96% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78 - 0℃; | 95% |

| Conditions | Yield |
|---|---|
| With iodine for 24h; Heating; | 95% |
| With trifluorormethanesulfonic acid at 60℃; for 16h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 95% |
| With diiodomethane at 100℃; for 120h; | 67% |
| With trimethylsilyl iodide at 80℃; Inert atmosphere; | 25% |
| With trifluorormethanesulfonic acid at 60℃; for 16h; Reagent/catalyst; Temperature; Time; Inert atmosphere; |
-
-
111-25-1
1-bromo-hexane

-
-
122-52-1
triethyl phosphite

-
A
-
78-40-0
triethyl phosphate

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

-
C
-
16165-66-5
diethyl hexylphosphonate

| Conditions | Yield |
|---|---|
| at 170℃; for 12h; Inert atmosphere; Dean-Stark; | A n/a B n/a C 95% |


| Conditions | Yield |
|---|---|
| With hydrogen; montmorillonite-(OSi(OMe)2CH2CH2PPh2)x In benzene at 100℃; under 25857.4 Torr; for 22h; | 90% |
| With ammonium formate; palladium on activated charcoal In methanol for 18h; Ambient temperature; | 88% |
| With hydrogen; <((t-Bu)2PH)PdP(t-Bu)2>2 (pretreated with oxygen) In tetrahydrofuran under 760 Torr; for 24h; Ambient temperature; | 75% |

| Conditions | Yield |
|---|---|
| With tetrachlorosilane at 0℃; | 90% |
| Stage #1: ethylphosphonic acid With 1H-imidazole; iodine In dichloromethane at 45 - 50℃; for 0.5h; Gareg-Samuelsson reaction; Stage #2: ethanol In dichloromethane at 45 - 50℃; for 0.75h; Gareg-Samuelsson reaction; | 90% |
| With p-TsOH-Celite at 20℃; | 86% |
-
-
100-51-6
benzyl alcohol

-
-
122-52-1
triethyl phosphite

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

-
B
-
1080-32-6
O,O-diethyl benzylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl alcohol; triethyl phosphite at 25℃; for 12h; Michaelis-Arbuzov Synthesis; Inert atmosphere; Schlenk technique; Sealed tube; Stage #2: With tetra-(n-butyl)ammonium iodide In neat (no solvent) at 125℃; for 24h; Michaelis-Arbuzov Synthesis; Inert atmosphere; Schlenk technique; Sealed tube; | A 12 %Chromat. B 90% |

-
-
78-94-4
methyl vinyl ketone

-
-
142778-06-1
diethyl (2-iodoethyl)phosphonate

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

-
B
-
1068-04-8
diethyl 5-oxo-n-hexylphosphonate

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; triethyl borane; tributyltin chloride In hexane; toluene at -78℃; Yields of byproduct given; | A n/a B 89% |

-
-
80436-45-9
diethyl (α-methylselenenylethyl)phosphonate

-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 1h; Heating; | 88% |

| Conditions | Yield |
|---|---|
| With sodium hypophosphite; air; triethyl borane In methanol; hexane at 20℃; for 2h; | 87% |
-
-
91210-95-6
α-lithioethyl diethylphosphonate

-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With oxonium | 84% |
-
-
85426-94-4
5-deoxy-5-iodo-1,2-O-isopropylidene-3-O-methyl-α-D-xylofuranose

-
-
122-52-1
triethyl phosphite

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

-
B
-
17968-56-8
5-deoxy-5-C-(diethoxyphosphinyl)-1,2-O-isopropylidene-3-O-methyl-α-D-xylofuranose

| Conditions | Yield |
|---|---|
| at 150℃; for 10h; | A n/a B 83% |

-
-
111-34-2
-butyl vinyl ether

-
-
191861-66-2
Diethyl α-iodoethylphosphonate

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With triethyl borane; tri-n-butyl-tin hydride In hexane; toluene at -78℃; Yields of byproduct given; | A n/a B 82% |

-
-
126-68-1
O,O,O-triethyl phosphorothioate

-
A
-
598-02-7
Diethyl phosphate

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

-
C
-
762-04-9
phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With magnesium monoperoxyphthalate hexahydrate In water at 25℃; for 24h; | A 10% B 81% C 9% |

-
-
108-05-4
vinyl acetate

-
-
142778-06-1
diethyl (2-iodoethyl)phosphonate

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

-
B
-
32421-69-5
diethyl 4-acetoxy-n-butylphosphonate

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; triethyl borane; tributyltin chloride In hexane; toluene at -78℃; Yields of byproduct given; | A n/a B 81% |

-
-
109-92-2
ethyl vinyl ether

-
-
191861-66-2
Diethyl α-iodoethylphosphonate

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; triethyl borane; tributyltin chloride In hexane; toluene at -78℃; Yields of byproduct given; | A n/a B 81% |

-
-
7305-61-5
ethyl hydrogen ethylphosphonate

-
-
21504-43-8
1,1-di-ethoxyprop-1-ene

-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| In benzene at 78℃; for 2h; | 80% |
-
-
73778-48-0
(+/-)-α-dimethylphosphorylethyl p-tolyl sulphide

-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| In toluene for 1h; Heating; | 80% |
-
-
615-41-8
2-iodochlorobenzene

-
-
122-52-1
triethyl phosphite

-
A
-
78-40-0
triethyl phosphate

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

-
C
-
28036-18-2
2-Chlorphenylphosphonsaeurediethylester

| Conditions | Yield |
|---|---|
| With Pd/C (30%) at 175℃; for 12h; Inert atmosphere; Dean-Stark; | A n/a B n/a C 80% |

-
-
100-51-6
benzyl alcohol

-
-
122-52-1
triethyl phosphite

-
A
-
945933-82-4
benzylphosphonic acid benzyl ethyl ester

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

-
C
-
1080-32-6
O,O-diethyl benzylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: benzyl alcohol; triethyl phosphite at 125℃; for 24h; Michaelis-Arbuzov Synthesis; Inert atmosphere; Schlenk technique; Sealed tube; Stage #2: With tetra-(n-butyl)ammonium iodide In neat (no solvent) at 125℃; for 24h; Michaelis-Arbuzov Synthesis; Inert atmosphere; Schlenk technique; Sealed tube; | A 6 %Chromat. B 9 %Chromat. C 80% |

-
-
64-17-5
ethanol

-
A
-
78-40-0
triethyl phosphate

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

-
C
-
762-04-9
phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With phosphorous; CuCl2bpy3 In N,N-dimethyl-formamide at 50℃; Electrolysis; | A 79.3% B 5.4% C 15.3% |
| With phosphorous; CuCl2bpy3 In acetonitrile at 50℃; Electrolysis; | A 55% B 15.5% C 29.5% |
| With phosphorous; CuCl2bpy3 at 50℃; Electrolysis; | A 28.5% B 17.5% C 16% |

-
-
1474-78-8
triethyl phosphonoformate

-
A
-
78-40-0
triethyl phosphate

-
B
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| at 395℃; for 8h; | A 79% B 8% |
-
-
111-83-1
1-bromo-octane

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

-
B
-
1068-07-1
diethyl octylphosphonate

| Conditions | Yield |
|---|---|
| With 1-bromo-octane at 110℃; for 24h; Yield given; | A n/a B 79% |
-
-
111-83-1
1-bromo-octane

-
-
122-52-1
triethyl phosphite

-
A
-
78-38-6
ethylphosphonic acid diethyl ester

-
B
-
1068-07-1
diethyl octylphosphonate

| Conditions | Yield |
|---|---|
| at 110℃; for 24h; | A n/a B 79% |

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
237735-38-5
(6E,2R,3S,5S)-8-[(t-butyldimethylsilyl)oxy]-2,6-dimethyl-5-[(triisopropylsilyl)oxy]-3-[(trimethylsilyl)oxy]-6-octenal

-
-
237735-39-6
(2E,4S,6S,7R,8RS)-1-[(t-butyldimethylsilyl)oxy]-9-(diethylphosphono)-3,7-dimethyl-4-[(triisopropylsilyl)oxy]-6-[(trimethylsilyl)oxy]-2-decaen-8-ol

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With tert.-butyl lithium In tetrahydrofuran; pentane at -78℃; for 0.75h; Stage #2: (6E,2R,3S,5S)-8-[(t-butyldimethylsilyl)oxy]-2,6-dimethyl-5-[(triisopropylsilyl)oxy]-3-[(trimethylsilyl)oxy]-6-octenal In tetrahydrofuran; pentane for 0.166667h; | 100% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide at 75℃; for 90h; | 99.9% |
| With phosphorus pentachloride; trichlorophosphate at 25℃; for 24h; | 90% |
| With thionyl chloride In N,N-dimethyl-formamide at 75℃; for 24h; | 53% |
-
-
886220-95-7
(1R,2S,3R,4S)-3-(tert-Butyl-dimethyl-silanyloxy)-4-methyl-1-trimethylsilanyloxy-2-vinyl-cyclopentanecarboxylic acid methyl ester

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
886220-87-7
{2-[(1R,2S,3R,4S)-3-(tert-Butyl-dimethyl-silanyloxy)-4-methyl-1-trimethylsilanyloxy-2-vinyl-cyclopentyl]-1-methyl-2-oxo-ethyl}-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; Stage #2: (1R,2S,3R,4S)-3-(tert-Butyl-dimethyl-silanyloxy)-4-methyl-1-trimethylsilanyloxy-2-vinyl-cyclopentanecarboxylic acid methyl ester In tetrahydrofuran; hexane at 0℃; for 1h; Claisen-type condensation; | 99% |
-
-
5765-65-1
4-(benzoyloxy)morpholine

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
1582788-23-5
diethyl (1-morpholinoethyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With bis(2,2,6,6-tetramethyl-1-piperidyl)zinc In toluene at 20℃; for 1h; Stage #2: 4-(benzoyloxy)morpholine With [2,2]bipyridinyl; copper dichloride In tetrahydrofuran; toluene at 20℃; for 4h; Catalytic behavior; Reagent/catalyst; Time; | 99% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
666856-39-9
(1R,2S,3R,4S)-3-(tert-Butyl-dimethyl-silanyloxy)-1-hydroxy-4-methyl-2-vinyl-cyclopentanecarboxylic acid methyl ester

-
-
886220-87-7
{2-[(1R,2S,3R,4S)-3-(tert-Butyl-dimethyl-silanyloxy)-4-methyl-1-trimethylsilanyloxy-2-vinyl-cyclopentyl]-1-methyl-2-oxo-ethyl}-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: chloro-trimethyl-silane; (1R,2S,3R,4S)-3-(tert-Butyl-dimethyl-silanyloxy)-1-hydroxy-4-methyl-2-vinyl-cyclopentanecarboxylic acid methyl ester With 1H-imidazole; dmap at 20℃; for 2h; Stage #2: ethylphosphonic acid diethyl ester In tetrahydrofuran at -78 - 20℃; | 98% |

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
103-25-3
3-phenylpropanoic acid methyl ester

-
-
1189399-12-9
(1-methyl-2-oxo-4-phenylbutyl)phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester; 3-phenylpropanoic acid methyl ester With lithium diisopropyl amide In tetrahydrofuran at -5 - 0℃; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water pH=ca. 4; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: C17H31BrO3Si In tetrahydrofuran; hexane at -78℃; for 1h; | 96% |
-
-
78-38-6
ethylphosphonic acid diethyl ester

-
A
-
75-00-3
chloroethane

-
B
-
1066-50-8
Ethylphosphonic dichloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; N,N-dimethyl-formamide for 18h; Heating; | A n/a B 95.8% |

| Conditions | Yield |
|---|---|
| With tetrabutyl phosphonium bromide at 180℃; for 20h; Reagent/catalyst; Inert atmosphere; | 95% |
-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
60456-21-5
methyl (4S)-2,2-dimethyl-1,3-dioxolane-4-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 2h; Stage #2: methyl (4S)-2,2-dimethyl-1,3-dioxolane-4-carboxylate In tetrahydrofuran; hexane for 1h; | 95% |
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 2h; Stage #2: methyl (4S)-2,2-dimethyl-1,3-dioxolane-4-carboxylate In tetrahydrofuran; hexane for 1h; | 95% |
-
-
82253-44-9
1,4-Dioxaspiro<4.4>nonan-6-essigsaeure-ethylester

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
82253-45-0
diethyl 1-methyl-2-oxo-3-<2-(1,3-dioxolano)cyclopentyl>propyl phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.55h; Inert atmosphere; Stage #2: 1,4-Dioxaspiro<4.4>nonan-6-essigsaeure-ethylester In tetrahydrofuran; hexane at -78 - 20℃; for 2h; Inert atmosphere; | 94% |
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.55h; Stage #2: 1,4-Dioxaspiro<4.4>nonan-6-essigsaeure-ethylester In tetrahydrofuran; hexane at -78 - 20℃; for 2h; | 94% |
| With n-butyllithium 1) THF, hexane, - 78 deg C, 0.55 h, 2) THF, hexane, -78 deg C, 1 h, then warm to room temp.; Yield given. Multistep reaction; |
-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane 1.) -78 deg C, 1 h, 2.) -78 deg C, 4 h; | 94% |
-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran for 1h; | 94% |
| With n-butyllithium In tetrahydrofuran; hexane 1.) -78 deg C, 50 min, 2.) -78 deg C, 50 min; | 94% |
-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
2216-51-5
(-)-menthol

-
-
930597-00-5
ethyl (-)-menthyl ethylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: (-)-menthol With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.166667h; Stage #2: ethylphosphonic acid diethyl ester In tetrahydrofuran; hexane at 20℃; | 94% |
-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
150765-78-9
2-methyl-3-oxocyclohex-1-en-1-yl trifluoromethanesulfonate

-
-
1225212-02-1
C13H23O4P

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.333333h; Inert atmosphere; Stage #2: 2-methyl-3-oxocyclohex-1-en-1-yl trifluoromethanesulfonate In tetrahydrofuran; hexane at -78 - 60℃; Inert atmosphere; Stage #3: With water; ammonium chloride In tetrahydrofuran; diethyl ether; hexane Inert atmosphere; | 94% |
-
-
5707-04-0
Phenylselenyl chloride

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
19894-83-8
ethyl (E)-5,9-dimethyl-4,8-decadienoate

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester; ethyl (E)-5,9-dimethyl-4,8-decadienoate With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -10℃; Inert atmosphere; Stage #2: Phenylselenyl chloride In tetrahydrofuran; hexane at -78℃; Inert atmosphere; | 93% |

-
-
78-38-6
ethylphosphonic acid diethyl ester

-
-
106-95-6
allyl bromide

-
-
71071-59-5
diethyl 1-methyl-n-but-3-enylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran at -78℃; for 1h; Metallation; Stage #2: allyl bromide In tetrahydrofuran at -78 - 20℃; Alkylation; | 92% |
| (i) nBuLi, (ii) CuI, (iii) /BRN= 605308/; Multistep reaction; | |
| With n-butyllithium 1.) THF, -78 deg C, 2.) THF, -78 to 25 deg C; Yield given. Multistep reaction; | |
| Stage #1: ethylphosphonic acid diethyl ester With n-butyllithium In tetrahydrofuran at -78℃; Metallation; Stage #2: allyl bromide Alkylation; |

| Conditions | Yield |
|---|---|
| at 160℃; for 11.3333h; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: ethylphosphonic acid diethyl ester With hydrogenchloride In water for 20h; Reflux; Stage #2: 4-nitrobenzyl chloride With pyridine; 2H-tetrazole; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 18h; | 92% |
-
-
913288-93-4
(2S,3S)-3-(tert-Butyl-dimethyl-silanyloxy)-2,5-dimethyl-hexanethioic acid S-{(1S,2R)-2-[benzyl-(2,4,6-trimethyl-benzenesulfonyl)-amino]-1-phenyl-propyl} ester

-
-
78-38-6
ethylphosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -78℃; | 91% |
Diethyl ethylphosphonate Specification
The Diethyl ethylphosphonate with CAS registry number of 78-38-6 is also known as Phosphonic acid,P-ethyl-, diethyl ester. The IUPAC name is 1-[Ethoxy(ethyl)phosphoryl]oxyethane. Its EINECS registry number is 201-111-9. In addition, the formula is C6H15O3P and the molecular weight is 166.16. This chemcial is a colorless liquid with a mild odor and it is used as efficient organic phosphorus flame retardant which can be added extensively in a variety of rigid foam.
Physical properties about Diethyl ethylphosphonate are: (1)ACD/LogP: 0.82; (2)ACD/LogD (pH 5.5): 0.82; (3)ACD/LogD (pH 7.4): 0.82; (4)ACD/BCF (pH 5.5): 2.46; (5)ACD/BCF (pH 7.4): 2.46; (6)ACD/KOC (pH 5.5): 66.29; (7)ACD/KOC (pH 7.4): 66.29; (8)#H bond acceptors: 3; (9)#Freely Rotating Bonds: 5; (10)Index of Refraction: 1.403; (11)Molar Refractivity: 40.21 cm3; (12)Molar Volume: 164.4 cm3; (13)Surface Tension: 29.2 dyne/cm; (14)Density: 1.01 g/cm3; (15)Flash Point: 92.7 °C; (16)Enthalpy of Vaporization: 41.64 kJ/mol; (17)Boiling Point: 198 °C at 760 mmHg; (18)Vapour Pressure: 0.518 mmHg at 25 °C.
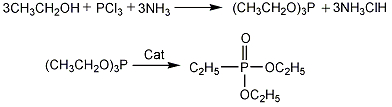
Preparation of Diethyl ethylphosphonate. Firstly, ethanol, methyl red are mixed with solvent and the mixture is added to the reactor. Then ammonia is passed into the reaction solution under stirring at the temperature of 0 °C. Dropping phosphorus trichloride slowly at the same time. The reaction needs 2-3 hours with the reaction temperature of 0-10 °C. Secondly, the reaction mixture is dissolved in water. After solvent recovery by vacuum distillation, the reservoir is vacuum distillated to collect triethyl phosphite at 52-53 °C with the yield of 70-75%. At last, product is obtained by isomerization reaction with catalyst.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable gloves and eye/face protection. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCOP(=O)(CC)OCC
2. InChI: InChI=1S/C6H15O3P/c1-4-8-10(7,6-3)9-5-2/h4-6H2,1-3H3
3. InChIKey: AATNZNJRDOVKDD-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 2500mg/kg (2500mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA | "Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 120, 1999. |
| rat | LD50 | oral | 2330mg/kg (2330mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA | "Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 120, 1999. |
Related Products
- Diethyl (1-phenylpropyl)malonate
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(diethoxymethylsilyl)ethyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (beta,gamma-epoxypropyl)phosphonate
- 78388-61-1
- 78389-19-2
- 78392-84-4
- 78-39-7
- 78-40-0
- 784-04-3
- 78407-21-3
- 78409-21-9
- 78409-50-4
- 784102-46-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View