-
Name
Dimethyl phosphite
- EINECS 212-783-8
- CAS No. 868-85-9
- Article Data99
- CAS DataBase
- Density 1.2g/mLat 25°C(lit.)
- Solubility Soluble in water.
- Melting Point
- Formula C2H7O3P
- Boiling Point 170.499 °C at 760 mmHg
- Molecular Weight 110.05
- Flash Point 29.444 °C
- Transport Information UN 3278
- Appearance colourless liquid
- Safety 26-36/37
- Risk Codes 21-36-10
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Dimethoxyphosphineoxide;Dimethyl acid phosphite;Dimethyl hydrogen phosphite;Dimethyl hydrogenphosphonate;Dimethyl phosphite;Dimethyl phosphonate;Hydrogen dimethylphosphite;Methyl phosphonate ((MeO)2HPO);
- PSA 59.00000
- LogP 0.66890
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In dichloromethane for 0.166667h; | 98% |
| With phosphorus trichloride | |
| With phosphorus trichloride |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; water at 25℃; Sealed tube; | 92% |
| With water In tetrahydrofuran at 20℃; Inert atmosphere; | 82% |
| With water In methanol | |
| With [Pt(H2O)2(1,1'-bis(diphenylphosphanyl)octamethylferrocene)](trifluoromethylsulfonate)2; water In tetrahydrofuran at 20℃; for 0.0833333h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | |
| With trifluorormethanesulfonic acid In water at 25℃; for 0.5h; Inert atmosphere; |
-
-
67-56-1
methanol

-
-
91157-34-5
Dimethyl 2-(N-tert-butyl-N-methylamino)benzoylphosphonate

-
B
-
868-85-9
Dimethyl phosphite

-
C
-
85-91-6
Methyl N-methylanthranilate

| Conditions | Yield |
|---|---|
| Heating; | A 90% B 30% C 5% |


| Conditions | Yield |
|---|---|
| With phosphorus trichloride In chloroform at 0℃; for 0.5h; | A 84% B 16% |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In chloroform at 25℃; for 0.5h; | A 83% B 3% C 14% |

-
-
18106-71-3
dimethyl benzoylphosphonate

-
A
-
613-94-5
benzoic acid hydrazide

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 0℃; for 0.5h; Title compound not separated from byproducts; | A 80% B n/a |
-
-
67-56-1
methanol

-
A
-
13598-36-2
phosphonic Acid

-
B
-
868-85-9
Dimethyl phosphite

-
C
-
13590-71-1
phosphonic acid monomethyl ester

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 25℃; for 1h; | A 1% B 73% C 26% |


| Conditions | Yield |
|---|---|
| With phosphorous; 10H-phenothiazine; N,N,N,N-tetraethylammonium tetrafluoroborate In N,N-dimethyl-formamide at 53℃; electrolysis; | A 9% B 71% |
-
-
67-56-1
methanol

-
A
-
512-56-1
trimethyl phosphite

-
B
-
868-85-9
Dimethyl phosphite

-
C
-
756-79-6
dimethyl methane phosphonate

| Conditions | Yield |
|---|---|
| With phosphorous; tetraethylammonium iodide In acetonitrile at 18℃; electrolysis; | A 70% B 11% C 3% |
| With phosphorous; tetraethylammonium iodide In acetonitrile at 18℃; Mechanism; Product distribution; electrolysis; var. nucleophiles; var. temperatures; | A 70% B 11% C 3% |
| With phosphorous In acetonitrile at 18℃; Electrolysis; | A 70% B 11% C 3% |

-
-
80094-07-1
dimethyl <4-(diphenylmethylene)-1,4-dihydro-1-hydroxynaphthyl>phosphonate

-
A
-
5690-41-5
4-(diphenylmethylene)-1(4H)-naphthalenone

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| under 1 Torr; Heating; | A 69% B n/a |
-
-
107-10-8
propylamine

-
-
18106-71-3
dimethyl benzoylphosphonate

-
A
-
10546-70-0
N-(n-propyl)benzamide

-
B
-
16115-01-8
α--α--toluol

-
C
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.05h; Ambient temperature; | A 68% B 27% C 7% |
| In diethyl ether for 0.05h; Product distribution; Ambient temperature; Substituent effect of alkyl groups of Benzoyl phosphonates in Benzoylation; | A 68% B 27% C 7% |

-
-
128967-40-8
(2-Oxo-3-phenylamino-2,3-dihydro-1H-indol-3-yl)-phosphonic acid dimethyl ester

-
A
-
33828-98-7, 101671-27-6
3-phenylimino-2-indolinone

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| at 230℃; under 5 Torr; for 0.5h; Product distribution; | A 55% B n/a |
-
-
18351-42-3
dimethyl phenylphosphonite

-
A
-
16390-98-0
methyl phenyl H-phosphonate

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; water at 25℃; Sealed tube; | A 30 %Spectr. B 50% |
-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
123-54-6
acetylacetone

-
A
-
1825-61-2
Methoxytrimethylsilane

-
B
-
25145-04-4
(Z)-4-trimethylsiloxypent-3-en-2-one

-
C
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| at 60 - 70℃; for 4h; Further byproducts given; | A 8.5% B n/a C 6.4% D 20.5% |

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
123-54-6
acetylacetone

-
A
-
1825-61-2
Methoxytrimethylsilane

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| at 60 - 70℃; for 4h; Further byproducts given; | A 8.5% B 6.4% C 20.5% |

-
-
36198-87-5
dimethyl trimethylsilyl phosphite

-
-
123-54-6
acetylacetone

-
A
-
1825-61-2
Methoxytrimethylsilane

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| at 60 - 70℃; for 4h; Further byproducts given; | A 8.5% B 6.4% C 20.5% D 9.2% |


| Conditions | Yield |
|---|---|
| With hypophosphorous acid Ambient temperature; |
-
-
110-91-8
morpholine

-
-
18106-71-3
dimethyl benzoylphosphonate

-
A
-
1468-28-6
4-benzoylmorpholine

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With water 1.) ether, room temperature, 24 h; 2.) THF, room temperature, 3 h; Yield given. Multistep reaction; |

-
-
51463-66-2
dimethyl (2-phenylacetyl)phosphonate

-
A
-
868-85-9
Dimethyl phosphite

-
B
-
937-39-3
2-phenylacetylhydrazine

| Conditions | Yield |
|---|---|
| With hydrazine |

| Conditions | Yield |
|---|---|
| Mechanism; var. aliphatic alcohols, their relative reaction rates; |
-
-
107-10-8
propylamine

-
-
18106-71-3
dimethyl benzoylphosphonate

-
A
-
10546-70-0
N-(n-propyl)benzamide

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With water 1.) ether, room temperature, 24 h; 2.) THF, room temperature, 3 h; Yield given. Multistep reaction; |

-
-
1641-40-3
pyrocatechol phosphorochloridite

-
-
51933-13-2
3,3-Diethoxy-butan-2-one

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
10072-02-3
2-ethoxy-1,3,2-benzodioxaphosphole

-
B
-
20570-25-6
2-methoxy-1,3,2-benzodioxaphosphole

-
C
-
144748-40-3
[1-(Benzo[1,3,2]dioxaphosphol-2-yloxy)-2-ethoxy-1-methyl-allyl]-phosphonic acid dimethyl ester

-
D
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| at 20 - 25℃; for 3h; Title compound not separated from byproducts; |

-
-
463-71-8
thiophosgene

-
-
116-17-6
triisopropyl phosphite

-
A
-
512-56-1
trimethyl phosphite

-
B
-
101632-67-1
C22H49O9P3S

-
C
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; allowed to warm to room temperature over 16h; Title compound not separated from byproducts; |

-
-
34881-14-6
dimethyl (9-hydroxy-9H-fluoren-9-yl) phosphonate

-
A
-
486-25-9
9-fluorenone

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With triethylamine Rate constant; other reagents (n-butyl- and t-butylamine); |
-
-
18106-71-3
dimethyl benzoylphosphonate

-
-
78-81-9
isobutylamine

-
A
-
5705-57-7
N-isobutyl-benzamide

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With water 1.) ether, room temperature, 24 h; 2.) THF, room temperature, 3 h; Yield given. Multistep reaction; |

-
-
122194-07-4
dimethyl N,N-diisopropylphosphoramidite

-
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With pyridine hydrochloride In pyridine; water for 0.0833333h; |
-
-
18106-71-3
dimethyl benzoylphosphonate

-
-
108-91-8
cyclohexylamine

-
A
-
1759-68-8
N-benzoylcyclohexylamine

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With water 1.) ether, room temperature, 24 h; 2.) THF, room temperature, 3 h; Yield given. Multistep reaction; |

-
-
18106-71-3
dimethyl benzoylphosphonate

-
-
104-15-4
toluene-4-sulfonic acid

-
A
-
13079-28-2
benzoic toluene-p-sulphonic anhydride

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| In benzene for 12h; Ambient temperature; |

-
-
18106-71-3
dimethyl benzoylphosphonate

-
-
104-15-4
toluene-4-sulfonic acid

-
A
-
868-85-9
Dimethyl phosphite

-
B
-
13590-71-1
phosphonic acid monomethyl ester

| Conditions | Yield |
|---|---|
| 1.) benzene, r.t., 12 h; 2.) benzene, 4 h, reflux; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
72304-75-7
dimethyl (phenoxycarbonyl)phosphonate

-
A
-
56317-55-6
sodium methyl hydrogen phosphite

-
B
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With water In acetonitrile for 0.0833333h; Mechanism; phosphate buffer pH 7.4; |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
868-85-9
Dimethyl phosphite

-
-
6329-48-2
dimethyl 1-hydroxy-1-(4-chlorophenyl)methylphosphonate

| Conditions | Yield |
|---|---|
| With triethylamine at 50℃; Pudovik Reaction; Inert atmosphere; Sealed tube; | 100% |
| Stage #1: 4-chlorobenzaldehyde With phosphotungstic acid supported on silica-coated magnetic Fe3O4 nanoparticle In neat (no solvent) at 20℃; for 0.25h; Kabachnik-Fields Reaction; Green chemistry; Stage #2: Dimethyl phosphite In neat (no solvent) at 20℃; for 4h; Green chemistry; | 96% |
| With potassium phosphate In neat (no solvent) at 20℃; for 0.0666667h; Green chemistry; | 93% |
-
-
100-52-7
benzaldehyde

-
-
868-85-9
Dimethyl phosphite

-
-
6329-46-0, 79296-54-1, 97995-93-2, 147731-04-2
dimethyl 1-hydroxy-1-phenylmethylphosphonate

| Conditions | Yield |
|---|---|
| With potassium fluoride for 0.333333h; | 100% |
| With potassium fluoride at 20℃; for 0.416667h; Inert atmosphere; | 100% |
| Stage #1: benzaldehyde With phosphotungstic acid supported on silica-coated magnetic Fe3O4 nanoparticle In neat (no solvent) at 20℃; for 0.25h; Kabachnik-Fields Reaction; Green chemistry; Stage #2: Dimethyl phosphite In neat (no solvent) at 20℃; for 6h; Solvent; Reagent/catalyst; Time; Green chemistry; | 98% |
-
-
86-81-7
3,4,5-trimethoxy-benzaldehyde

-
-
868-85-9
Dimethyl phosphite

-
-
130190-35-1
dimethyl α-hydroxy-α-(3,4,5-trimethoxyphenyl)methanephosphonate

| Conditions | Yield |
|---|---|
| With dibutylamine In diethyl ether at 20℃; for 3h; | 100% |
| With sodium methylate In methanol at 50℃; | 96.5% |
| With methanol; sodium for 0.5h; Heating; | 90% |
-
-
111-50-2
Adipic acid dichloride

-
-
868-85-9
Dimethyl phosphite

-
-
121-45-9
phosphorous acid trimethyl ester

| Conditions | Yield |
|---|---|
| at 130 - 135℃; for 6h; | 100% |


| Conditions | Yield |
|---|---|
| at 60℃; for 8h; | 100% |


| Conditions | Yield |
|---|---|
| at 130 - 135℃; for 6h; | 100% |

-
-
121-44-8
triethylamine

-
-
868-85-9
Dimethyl phosphite

-
-
122833-31-2
phosphonic acid monomethyl ester methyltriethylammonium salt

| Conditions | Yield |
|---|---|
| at 70℃; for 6h; | 100% |
| In methanol for 2h; Heating; |

| Conditions | Yield |
|---|---|
| at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| at 60℃; for 8h; | 100% |


| Conditions | Yield |
|---|---|
| at 130 - 135℃; for 6h; | 100% |


| Conditions | Yield |
|---|---|
| at 130 - 135℃; for 6h; | 100% |


| Conditions | Yield |
|---|---|
| at 130 - 135℃; for 6h; | 100% |


| Conditions | Yield |
|---|---|
| In methanol at 50℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| at 5℃; for 24h; | 100% |
-
-
50-00-0
formaldehyd

-
-
868-85-9
Dimethyl phosphite

-
-
24630-67-9
hydroxymethyl-phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 65℃; | 100% |
| With potassium carbonate In methanol at 20℃; for 1h; Pudovik reaction; | 98% |
| 83% |
-
-
18031-40-8
(S)-(-)-perillaldehyde

-
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 24h; Ambient temperature; | 100% |
-
-
100-52-7
benzaldehyde

-
-
868-85-9
Dimethyl phosphite

-
-
62-53-3
aniline

-
-
26624-91-9
dimethyl (phenyl)(phenylamino)methylphosphonate

| Conditions | Yield |
|---|---|
| With tin(ll) chloride at 20℃; under 760.051 Torr; for 2.5h; Kabachnik-Fields reaction; neat (no solvent); | 100% |
| at 80℃; for 0.0333333h; Product distribution; Further Variations:; Temperatures; microwave irradiation; Kobachnik-Fields reaction; microwave irradiation; | 98% |
| With zirconium(IV) oxychloride at 20℃; for 0.0833333h; | 98% |

-
-
868-85-9
Dimethyl phosphite

-
-
740805-26-9
(3S,4R,5S)-3,4-isopropylidenedioxy-5-methyl-1-pyrroline-N-oxide

| Conditions | Yield |
|---|---|
| at 40℃; for 20h; | 100% |
-
-
459-57-4
4-fluorobenzaldehyde

-
-
868-85-9
Dimethyl phosphite

-
-
183240-18-8
(+/-)-dimethyl hydroxy(4-fluorophenyl)methylphosphonate

| Conditions | Yield |
|---|---|
| With dibutylamine In diethyl ether at 20℃; for 3h; | 100% |
| With triethylamine In acetone for 0.166667h; Pudovik Reaction; Reflux; | 96% |
| With guanidine supported magnetic Fe3O4 nanoparticle In neat (no solvent) at 80℃; for 3.5h; | 85% |
-
-
82113-65-3
bis(trifluoromethanesulfonyl)amide

-
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 3h; | 100% |
-
-
14385-49-0
8-(4'-methoxyphenoxy)acetophenone

-
-
868-85-9
Dimethyl phosphite

-
-
4202-12-4
1-phenylvinyl dimethyl phosphate

| Conditions | Yield |
|---|---|
| With caesium carbonate In dichloromethane at 20℃; Inert atmosphere; | 100% |

-
-
868-85-9
Dimethyl phosphite

-
-
1435940-00-3
dimethyl (2-(4-bromophenyl)-1,2,3,4-tetrahydroisoquinolin-1-yl)phosphonate

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; cobalt(II) acetate hydrate In acetonitrile at 80℃; for 16h; | 100% |
| With 10-methylacridine-3(10H)-one In methanol for 11h; Irradiation; | 78% |
| With oxygen In 1,4-dioxane at 80℃; for 30h; | 67% |
| With C44Cl8F20N4Pd; oxygen; potassium carbonate In methanol; acetonitrile for 3h; UV-irradiation; | 76 %Spectr. |

| Conditions | Yield |
|---|---|
| at 25℃; for 1h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In benzene-d6 at 20℃; for 15h; Glovebox; Inert atmosphere; | 100% |
-
-
13657-16-4
3-benzyl-1,3-oxazolidine

-
-
868-85-9
Dimethyl phosphite

-
-
73215-14-2
dimethyl <methyl>phosphonate

| Conditions | Yield |
|---|---|
| at 30 - 40℃; for 2h; | 99.6% |

| Conditions | Yield |
|---|---|
| With dibenzo-24-crown ether In benzene for 4h; Heating; | 99% |


| Conditions | Yield |
|---|---|
| With dibenzo-24-crown ether In benzene for 4h; Heating; | 99% |


| Conditions | Yield |
|---|---|
| With triethylamine In pentane for 72h; Ambient temperature; | 99% |
-
-
103697-17-2
(4-iodophenyl)phosphonate de diethyle

-
-
868-85-9
Dimethyl phosphite

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In ammonia for 0.333333h; Irradiation; | 99% |
-
-
100-52-7
benzaldehyde

-
-
868-85-9
Dimethyl phosphite

-
-
97995-93-2
dimethyl (S)-phenyl(hydroxy)methylphosphonate

| Conditions | Yield |
|---|---|
| Stage #1: benzaldehyde; Dimethyl phosphite With C41H64AlClN2O2; potassium carbonate In diethyl ether at -30℃; for 24h; Stage #2: With hydrogenchloride; water In diethyl ether optical yield given as %ee; enantioselective reaction; | 99% |
| With C38H48N4P(1+)*Cl(1-); potassium tert-butylate In tetrahydrofuran at -98℃; for 4h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 97% |
| (R)-LaLi3(binaphthoxide)3 In tetrahydrofuran at -78℃; for 8h; Yield given; |
Dimethyl phosphonate Consensus Reports
Dimethyl phosphonate Standards and Recommendations
Dimethyl phosphonate Specification
The Dimethyl phosphonate, with the CAS registry number 868-85-9 and EINECS registry number 212-783-8, is also called phosphonic acid, dimethyl ester. It is a kind of colourless liquid, and belongs to the following product categories: Pharmaceutical Intermediates; Organic Building Blocks; Organic Phosphates/Phosphites; Phosphorus Compounds. And the molecular formula of this chemical is C2H7O3P. What's more, It is used intermediate in production of insecticides or herbicides, and it is also used as organophosphorus pesticides dipterex and herbicide glyphosate.
The physical properties of Dimethyl phosphonate are as following: (1)ACD/LogP: -1.28; (2)# of Rule of 5 Violations: 0; (3)#H bond acceptors: 3; (4)#H bond donors: 0; (5)#Freely Rotating Bonds: 2; (6)Polar Surface Area: 59 Å2; (7)Flash Point: 29.444 °C; (8)Enthalpy of Vaporization: 39.018 kJ/mol; (9)Boiling Point: 170.499 °C at 760 mmHg; (10)Vapour Pressure: 1.945 mmHg at 25°C.
Preparation of Dimethyl phosphonate: It can be prepared by methanol and phosphorus trichloride:
3CH3OH+PCl3→(CH3O)2POH+2HCl+CH3Cl
Uses of Dimethyl phosphonate: It can react with cyclohexanone to produce (1-hydroxy-cyclohexyl)-phosphonic acid dimethyl ester. This reaction will need catalyst 1,5,7-triazabicyclo[4.4.0]dec-5-ene with temperature of 0°C, and the yield is about 78%.
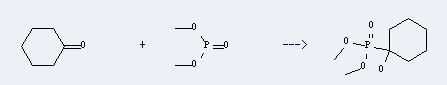
You should be cautious while dealing with this chemical. It is a kind of flammable chemical, and irritates eyes, and it is also harmful in contact with skin. Therefore, you had better take the following instructions: Wear suitable protective clothing and gloves, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=P(OC)OC
(2)InChI: InChI=1/C2H7O3P/c1-4-6(3)5-2/h6H,1-2H3
(3)InChIKey: HZCDANOFLILNSA-UHFFFAOYAF
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC | inhalation | > 7100mg/m3/6H (7100mg/m3) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BLOOD: HEMORRHAGE | National Technical Information Service. Vol. OTS0537047, |
| guinea pig | LD50 | oral | 900mg/kg (900mg/kg) | AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 36(4), Pg. 22, 1992. |
| mouse | LC | inhalation | > 7100mg/m3/6H (7100mg/m3) | SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Technical Information Service. Vol. OTS0537047, |
| mouse | LD50 | oral | 1831mg/kg (1831mg/kg) | AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 36(4), Pg. 22, 1992. |
| mouse | LD50 | subcutaneous | 2610mg/kg (2610mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 36(4), Pg. 22, 1992. | |
| rabbit | LD50 | skin | 681mg/kg (681mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA | National Technical Information Service. Vol. OTS0537044, |
| rat | LC50 | inhalation | > 20gm/m3 (20000mg/m3) | Albright & Wilson Inc. Vol. #OPB-3, Pg. 1984, | |
| rat | LD50 | oral | 3040mg/kg (3040mg/kg) | National Toxicology Program Technical Report Series. Vol. NTP-TR-287, Pg. 1985, | |
| rat | LD50 | subcutaneous | 2970mg/kg (2970mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 36(4), Pg. 22, 1992. |
Related Products
- Dimethyl ( )-2,3-O-Isopropylidene-D-tartrate
- Dimethyl ((1-methyl-5-nitro-1H-imidazol-2-yl)methylene)propanedioate
- Dimethyl (2-oxo-3,3-difluoroheptyl)phosphonate
- Dimethyl (2-oxo-4-phenylbutyl)phosphonate
- Dimethyl (2-oxoheptyl)phosphonate
- Dimethyl (2S, 2'S)-1, 1'-((2S, 2'S)-2, 2'-(4, 4'-(biphenyl-4, 4'-diyl)bis(1H-imidazole-4, 2-diyl))bis(pyrrolidine-2, 1-diyl))bis(3-methyl-1-oxobutane-2, 1-diyl)dicarbamate
- Dimethyl (3-phenoxy-2-oxopropyl)phosphonate
- Dimethyl (R)-(+)-methylsuccinate
- Dimethyl (S)-(-)-methylsuccinate
- Dimethyl (S)-3-hydroxy-L-aspartate
- 86889-04-5
- 86891-03-4
- 86893-19-8
- 868944-72-3
- 868944-75-6
- 86895-09-2
- 86895-14-9
- 86901-30-6
- 86902-27-4
- 86904-44-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View