-
Name
Diphenyl ether
- EINECS 202-981-2
- CAS No. 101-84-8
- Article Data393
- CAS DataBase
- Density 1.063 g/cm3
- Solubility Insoluble in water
- Melting Point 26 °C
- Formula C12H10O
- Boiling Point 258.3 °C at 760 mmHg
- Molecular Weight 170.211
- Flash Point 99.3 °C
- Transport Information UN 3077 9/PG 3
- Appearance clear pale yellowish liquid after melting
- Safety 60-61-57-37/39-26-45-36/37
- Risk Codes 51/53-36/37/38-39/23/24/25-23/24/25-36/38
-
Molecular Structure
-
Hazard Symbols
 N,
N, Xi,
Xi, T
T
- Synonyms Phenylether (8CI);1,1'-Oxybis[benzene];Barrel Therm 330;Benzene, phenoxy-;Biphenyl oxide;Chemcryl JK-EB;Diphenyl oxide;NSC 19311;Oxybisbenzene;Phenoxybenzene;Phenyl oxide;Benzene, 1,1'-oxybis-;
- PSA 9.23000
- LogP 3.47890
Synthetic route
-
-
83566-43-2
tetraphenyl-bismuth trifluoroacetate

-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With phenol In benzene refluxing overnight (Ar); t.l.c.; | 100% |
| In benzene 24 h at 80°C; filtn., evapn., addn. of Cl3CCO2H in CH2Cl2, reflux for 2 h, cooling, washing, drying, t.l.c.; | 38% |
| With water In water; benzene 24 h at 80°C, addn. of water; filtn., evapn., addn. of Cl3CCO2H in CH2Cl2, reflux for 2 h, cooling, washing, drying., t.l.c.; | 34% |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; caesium carbonate; hydroxybenzaldoxime; 3 A molecular sieve In acetonitrile at 82℃; for 24h; Ullmann-type coupling; | 100% |
| With caesium carbonate; copper(I) oxide; trans-N,N'-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine In acetonitrile at 82℃; for 24h; Conversion of starting material; activated 3 A molecular sieve (KnNa12-n[(AlO2)12(SiO2)12]); | 100% |
| Stage #1: phenol With caesium carbonate; copper(I) oxide; trans-N,N'-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine at 100℃; Molecular sieve 3 Å; Stage #2: iodobenzene In acetonitrile at 82℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In benzene Heating; | 100% |
| In benzene at 80℃; for 12h; | 100% |

-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In tetrahydrofuran for 3h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; Raney Ni-Al alloy In 1,4-dioxane at 110℃; for 24h; | 99% |
| With caesium carbonate; copper(l) iodide In N,N-dimethyl-formamide at 110℃; for 18h; Ullmann condensation; | 98% |
| With 2-[(dimethylamino)methyl]-1-thiophenolato-copper(I); caesium carbonate In 1-methyl-pyrrolidin-2-one at 160℃; for 16h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With C60H48BP3Pd; potassium formate; [2.2.2]cryptande In tetrahydrofuran at 60℃; for 72h; Schlenk technique; Inert atmosphere; | 99% |
| With N-tert-butylaminoborane; palladium on activated charcoal In methanol at 20℃; for 120h; | 82% |
| With tributyl-amine; tetrabutylammonium tetrafluoroborate In acetonitrile at 20℃; for 1.8h; Inert atmosphere; Electrolysis; | 80% |
| With C8K In tetrahydrofuran Product distribution; Ambient temperature; | |
| With N-ethyl-N,N-diisopropylamine In acetonitrile for 16h; Irradiation; | 95 %Spectr. |
-
-
144150-76-5
4-phenoxyphenyl 4-methylbenzenesulfonate

-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In methanol; chloroform ice-cooling; | 94% |
| With sodium tetrahydroborate; bis(triphenylphosphine)nickel(II) chloride; tricyclohexylphosphine In tetrahydrofuran at 60℃; for 14h; | 70% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 120℃; for 5h; Ullmann diaryl etherification; Inert atmosphere; | 93% |
| With 1-butyl-3-methylimidazolium Tetrafluoroborate; copper(l) chloride at 100℃; for 10h; | 72% |
| With copper at 180 - 190℃; | |
| With copper at 210 - 230℃; |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 120℃; for 4h; Ullmann diaryl etherification; Inert atmosphere; | 93% |
| With 1-butyl-3-methylimidazolium Tetrafluoroborate; copper(l) chloride at 100℃; | 82% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 50℃; for 6h; | 93% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; (2,2'-bipyridyl)(1,5-cyclooctadiene)nickel In tetrahydrofuran at 55℃; for 48h; | A 3% B 92% |
| With 3-Hydroxy-1-methylpiperidine; nickel diacetate; sodium hydride In tetrahydrofuran at 65℃; for 0.5h; | A 2% B 81% |
| With lithium aluminium tetrahydride; (2,2'-bipyridyl)(1,5-cyclooctadiene)nickel In tetrahydrofuran at 55℃; for 48h; | A 79% B 3% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; palladium diacetate; triphenylphosphine In isopropyl alcohol at 81.9℃; for 5h; Product distribution; Mechanism; other aromatic mono- and polyhalides, var. solvents, transition metal salt, ligands, temperature, and reaction time; | 92% |
| With methanol; gold; hydrogen; caesium carbonate at 100℃; under 3800.26 Torr; for 96h; | 89% |
| With cadmium selenide; triethylamine In N,N-dimethyl-formamide at 20℃; for 24h; Irradiation; Sealed tube; | 54% |
| With 2 wt.% Pd/TiO2 In tetrahydrofuran; methanol for 1h; Catalytic behavior; Kinetics; Reagent/catalyst; UV-irradiation; Inert atmosphere; Darkness; | 15.7% |
| With borane-ammonia complex In water; isopropyl alcohol at 50℃; for 12h; Sealed tube; | 97 %Chromat. |
-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
108-95-2
phenol

-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 20℃; for 24h; | 92% |
| With cesium fluoride In acetonitrile at 20℃; for 24h; | 92% |
| With tetrabutyl ammonium fluoride In acetonitrile for 48h; Ambient temperature; | 38% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide for 0.133333h; Reagent/catalyst; Solvent; Time; Reflux; Microwave irradiation; | 92% |
-
-
144930-50-7
mesityl(phenyl)iodonium triflouromethanesulfonate

-
-
98-80-6
phenylboronic acid

-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With Eosin Y; sodium t-butanolate In N,N-dimethyl-formamide at 20℃; for 2h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Time; Irradiation; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In tetrahydrofuran at 115℃; for 18h; | 91% |
| With 1-butyl-3-methylimidazolium Tetrafluoroborate; copper(l) chloride at 100℃; | 76% |

| Conditions | Yield |
|---|---|
| With pyridine; copper(II) acetate monohydrate In dichloromethane at 20℃; for 16h; Temperature; | 91% |
| With copper diacetate; triethylamine In dichloromethane at 0℃; for 6h; Molecular sieve; | 85% |
| With potassium acetate In N,N-dimethyl-formamide at 20℃; for 15h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: phenol With sodium In diethyl ether Stage #2: 5-phenylthianthrenium bromide In acetonitrile at 80℃; for 48h; | A 3.8 % Chromat. B 6.7 % Chromat. C 90% D 2.9 % Chromat. |

-
-
1374791-08-8
2-(4-phenoxyphenoxy)pyridine

-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); sodium isopropylate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In tetrahydrofuran at 60℃; for 2h; Schlenk technique; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| copper In dichloromethane for 5h; Ambient temperature; | 88% |
-
-
101-84-8
diphenylether

-
-
93952-05-7
diphenyl ether-d10

| Conditions | Yield |
|---|---|
| With water-d2; isopropyl alcohol In n-heptane at 120℃; Flow reactor; | 100% |
| platinum(IV) oxide In water-d2 at 250℃; under 30002.4 Torr; for 12h; | 67% |
-
-
101-84-8
diphenylether

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In 1,2-dichloro-ethane Inert atmosphere; Schlenk technique; | 100% |

| Conditions | Yield |
|---|---|
| With isopropyl alcohol at 150℃; under 7500.75 Torr; for 12h; Inert atmosphere; Autoclave; | A 25.1% B 100% C 74.9% |
| With isopropyl alcohol at 160℃; for 15h; Autoclave; Inert atmosphere; | |
| With isopropyl alcohol at 150℃; for 10h; Catalytic behavior; Reagent/catalyst; Temperature; Sealed tube; | A 24.6 %Chromat. B 47.8 %Chromat. C 24.3 %Chromat. |
| With isopropyl alcohol at 150℃; for 6h; Temperature; Sealed tube; | A 15.2 %Chromat. B 17.7 %Chromat. C 5.8 %Chromat. |


| Conditions | Yield |
|---|---|
| Stage #1: diphenylether; 2-isopropylthioxanthone sulphoxide With sulfuric acid; acetic anhydride; acetic acid In dichloromethane at 15 - 20℃; for 2h; Stage #2: With potassium hexafluorophosphate; acetic acid In water | 99.3% |

| Conditions | Yield |
|---|---|
| With hydrogen In dodecane at 300℃; under 45004.5 Torr; for 1h; Autoclave; | 99.3% |
| With hafnium tetrakis(trifluoromethanesulfonate); Ru/Al2O3; hydrogen In octane at 250℃; under 30003 Torr; for 2h; Sealed tube; | 94.3% |
| With hydrogen In dodecane at 200℃; under 15001.5 Torr; for 2h; | 92% |

| Conditions | Yield |
|---|---|
| With bromine; NaBrO3 In 1,2-dichloroethane (EDC); water; 1,2-dichloro-ethane | 99% |
| With bromine In 1,2-dichloro-ethane at 0 - 20℃; for 18h; | 99% |
| With copper(II) carbonate; bromine In acetic acid for 2h; | 98% |
-
-
101-84-8
diphenylether

-
-
28896-49-3
bis(4-iodophenyl) ether

| Conditions | Yield |
|---|---|
| With poly; iodine In ethyl acetate at 60℃; for 16h; | 99% |
| With poly[4-(diacetoxyiodo)styrene]; iodine In ethyl acetate at 60℃; for 16h; Iodination; | 99% |
| With nitrosylsulfuric acid; nitromethane; iodine at 20℃; for 3h; | 98% |
-
-
101-84-8
diphenylether

-
-
20241-57-0
para-phenoxybenzenesulfonic acid

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In dichloromethane at 0℃; for 2h; | 99% |
| With sulfuric acid; acetic anhydride | |
| With sulfuric acid at 80℃; for 1h; Yield given; |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In neat (no solvent) at 20℃; for 1h; Cooling with ice; | 99% |
| With aluminium trichloride In nitromethane at 50 - 60℃; for 2h; | 49% |
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; iron(III) chloride In acetonitrile at 25℃; for 0.5h; | 99% |
| With iodine; Selectfluor In acetonitrile at 22℃; for 3h; | 80% |
| With ammonium iodide; air; nitrosonium tetrafluoroborate; trifluoroacetic anhydride In trifluoroacetic acid at 25℃; for 50h; | 74% |

| Conditions | Yield |
|---|---|
| With pyridin-2-yl trifluoromethanesulfonate; trifluoroacetic acid for 5h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane for 16h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With bis(acetylacetonate)nickel(II); cetyltrimethylammonim bromide; lithium tri-t-butoxyaluminum hydride; sodium t-butanolate; tricyclohexylphosphine In toluene at 70℃; for 5h; Micellar solution; | A 99% B 99% |
| With Ru0.6Ni0.4; hydrogen In water at 95℃; under 760.051 Torr; for 16h; Reagent/catalyst; | A 92% B 96% |
| With hydrogen In water at 110℃; under 7500.75 Torr; for 1h; Autoclave; |
-
-
101-84-8
diphenylether

-
-
790636-25-8
cis,cis,cis-1,2,3,4,5,6,7,8,9-nonachloro-10-azatricyclo[5.2.1.01,10]deca-2,5,8-triene

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane | 99% |

| Conditions | Yield |
|---|---|
| With ammonium nitrate; N-Bromosuccinimide In acetonitrile for 0.5h; | 98% |
| With N-Bromosuccinimide In acetonitrile at 0 - 23℃; for 16h; | 97% |
| With dimethylbromosulphonium bromide In dichloromethane for 8h; Ambient temperature; | 94% |

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; bromine for 3h; Heating; | 98% |
| With aluminum tri-bromide; bromine | |
| Stage #1: diphenylether With bromine at 25 - 58℃; for 1.66667 - 2.13333h; Stage #2: With bromine; aluminum (III) chloride at 55 - 60℃; for 6.38333 - 6.5h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| aluminum (III) chloride In orthodichlorobenzene at 50℃; for 3h; Product distribution / selectivity; | 98% |
| aluminum (III) chloride In 1,2-dichloro-ethane at 40℃; for 3h; Product distribution / selectivity; | 88% |
| antimonypentachloride; N-benzyl-N,N,N-triethylammonium chloride In nitromethane for 0.5h; Friedel-Crafts acylation; Heating; | 67% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 20℃; Friedel-Crafts acylation; | 98% |
| With aluminium trichloride In carbon disulfide Friedel-Crafts acylation; | 90% |
| With aluminum (III) chloride In dichloromethane at 0℃; for 24h; Friedel-Crafts Acylation; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: iodobenzene With peracetic acid; acetic acid In dichloromethane; 2,2,2-trifluoroethanol at 35℃; for 1h; Stage #2: diphenylether; toluene-4-sulfonic acid In dichloromethane; 2,2,2-trifluoroethanol at 20℃; for 3h; | 98% |

Diphenyl ether History
Diphenyl ether(CAS NO.101-84-8) and many of its properties were first reported as early as 1901.
Diphenyl ether Consensus Reports
Diphenyl ether and many of its properties were first reported as early as 1901.It is synthesized by a modification of the Williamson ether synthesis, here the reaction of phenol and bromobenzene in the presence of base and a catalytic amount of copper:
PhONa + PhBr → PhOPh + NaBr
Involving similar reactions, diphenyl ether is a significant side product in the high-pressure hydrolysis of chlorobenzene in the production of phenol
Diphenyl ether Specification
The Diphenyl ether with CAS registry number of 101-84-8 is also known as Benzene, phenoxy-. The IUPAC name is Phenoxybenzene. It belongs to product categories of Pharmaceutical Intermediates; Fine Chemical & Intermediates; Organics; Biphenyl & Diphenyl ether. Its EINECS registry number is 202-981-2. In addition, the formula is C12H10O and the molecular weight is 170.21. This chemical is a clear pale yellowish liquid after melting and insoluble in water. It should be sealed in ventilated and dry place away from fire, heat, oxidants. What's more, it can be used as heat transfer medium and used for organic synthesis, manufacture of perfume and dyes.
Physical properties about Diphenyl ether are: (1)ACD/LogP: 4.21; (2)ACD/LogD (pH 5.5): 4.21; (3)ACD/LogD (pH 7.4): 4.21; (4)ACD/BCF (pH 5.5): 932.39; (5)ACD/BCF (pH 7.4): 932.39; (6)ACD/KOC (pH 5.5): 4647.71; (7)ACD/KOC (pH 7.4): 4647.71; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 2; (10)Index of Refraction: 1.572; (11)Molar Refractivity: 52.69 cm3; (12)Molar Volume: 160 cm3; (13)Surface Tension: 38.4 dyne/cm; (14)Density: 1.063 g/cm3; (15)Flash Point: 99.3 °C; (16)Enthalpy of Vaporization: 47.59 kJ/mol; (17)Boiling Point: 258.3 °C at 760 mmHg; (18)Vapour Pressure: 0.0223 mmHg at 25 °C.
Preparation of Diphenyl ether: it is prepared by condensation reaction of chlorobenzene and phenol. The reaction needs catalyst copper and solvent caustic solution. When the reaction is complete, reactant is dealed with acid and the ether oil is vacuum distilled to obtain product.
(1) C6H5OH + NaOH → C6H5ONa + H2O
(2) C6H5ONa + C6H5Cl → C6H5OC6H5 + NaCl
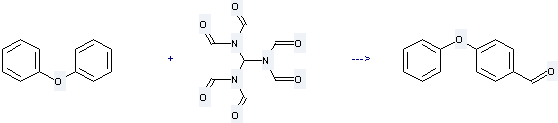
Uses of Diphenyl ether: it is used to produce 4-phenoxy-benzaldehyde by reaction with tris(diformylamino)methane. The reaction occurs with reagent AlCl3 and solvent 1,2-dichloro-ethane at -13 - 1 °C for 16 hours. The yield is about 20%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. There is danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. It also is toxic to aquatic organisms that may cause long-term adverse effects in the aquatic environment. During using it, wear suitable protective clothing, gloves and eye/face protection. Avoid release to the environment and use appropriate containment to avoid environmental contamination. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately. This material and its container must be disposed of as hazardous waste.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)OC2=CC=CC=C2
2. InChI: InChI=1S/C12H10O/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h1-10H
3. InChIKey: USIUVYZYUHIAEV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | > 7940mg/kg (7940mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | National Technical Information Service. Vol. 0TS0518143, |
| rat | LD50 | oral | 2450mg/kg (2450mg/kg) | BEHAVIORAL: FOOD INTAKE (ANIMAL) BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: OTHER CHANGES | National Technical Information Service. Vol. 0TS0518143, |
Related Products
- Diphenyl carbonate
- Diphenyl chlorophosphate
- Diphenyl diselenide
- Diphenyl disulfide
- Diphenyl ether
- Diphenyl isononyl phosphinate
- Diphenyl isophthalate
- Diphenyl N-cyanocarbonimidate
- Diphenyl phosphate
- Diphenyl Phosphite
- 10184-98-2
- 101852-89-5
- 101854-42-6
- 10185-46-3
- 10185-62-3
- 10185-65-6
- 10185-68-9
- 101859-99-8
- 101-86-0
- 101860-51-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View