-
Name
Diphenyl disulfide
- EINECS 212-926-4
- CAS No. 882-33-7
- Article Data1314
- CAS DataBase
- Density 1.22 g/cm3
- Solubility insoluble in water
- Melting Point 58-60 °C
- Formula C12H10S2
- Boiling Point 310 °C at 760 mmHg
- Molecular Weight 218.343
- Flash Point 177.8 °C
- Transport Information UN 3335
- Appearance White to light yellow crystal
- Safety 26-37/39-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Phenyldisulfide (8CI);1,1'-Diphenyl disulfide;Biphenyl disulfide;Diphenyldisulfide;Diphenyl disulphide;NSC 2689;
- PSA 50.60000
- LogP 4.48600
Synthetic route

| Conditions | Yield |
|---|---|
| With ethyl phosphinate In ethanol other reagent; | 100% |
| With ethyl phosphinate In ethanol Product distribution; other reagent; | 100% |
| With tungsten(VI) chloride; sodium iodide In acetonitrile for 1.5h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With pyridine chromium peroxide for 0.1h; Product distribution; effect of various chromium(VI) based oxidants; | 100% |
| With bis(tetra-n-butylammonium) tetrakis(benzenethiolato-μ3-sulfidoiron); oxygen In acetonitrile at 0℃; for 0.166667h; other iron-sulfur complex or other iron compounds as the catalyst in reaction oxidation; | 100% |
| With barium ferrate(VI) In benzene for 0.25h; Product distribution; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide In dichloromethane at 25℃; for 16h; | 100% |
| With tungsten(VI) chloride; sodium iodide In acetonitrile for 16h; Ambient temperature; | 97% |
| With Silphos; iodine In acetonitrile for 10h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol | A 100% B 61% |

| Conditions | Yield |
|---|---|
| With (2,2,4,4-tetrafluoropropoxy)tellurane In diethyl ether at -50 - -40℃; for 0.166667h; | A 100% B n/a |
-
-
93-59-4
Perbenzoic acid

-
-
24364-84-9
bis-benzenesulfenyl-amine

-
A
-
93-99-2
benzoic acid phenyl ester

-
B
-
92-52-4
biphenyl

-
C
-
65-85-0
benzoic acid

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In benzene Kinetics; Product distribution; Mechanism; isotopic effect, effect of benzoic acid and styrene on the reaction; | A 1.4% B 0.2% C 100% D 50% E n/a |


| Conditions | Yield |
|---|---|
| With ethanol In dichloromethane CH2Cl2 soln. of reagents was stirred at 0°C for 1 h under N2; concd., chromd. on silica gel with hexane-ethyl acetate; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| In dichloromethane CH2Cl2, 0°C, 1 h; | A 100% B n/a |

-
-
108-98-5
thiophenol

-
-
233257-02-8
[(trifluoroacetyl)imino]tris-(2-methylphenyl)-λ(5)-bismuthane

-
A
-
10050-08-5
tri-o-tolylbismuth

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In benzene room temp., 3 h; | A 100% B 80% |

-
-
52777-99-8
N-benzylidene-S-phenylthiohydroxylamine

-
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) bis(trifluoromethanesulfonate) In toluene at 80℃; for 3h; | 100% |
-
-
618-32-6
Benzoyl bromide

-
-
57340-80-4
thallium thiophenoxide

-
A
-
884-09-3
phenyl thiobenzoate

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.5h; | A 99% B 1% |

-
-
110637-93-9, 58324-83-7
p-(1-methyl-1-nitropropyl)nitrobenzene

-
-
930-69-8
sodium thiophenolate

-
A
-
110637-97-3
2-(p-nitrophenyl)-2-butyl phenyl sulfide

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 0.75h; | A 99% B 99% |

-
-
57340-80-4
thallium thiophenoxide

-
-
98-88-4
benzoyl chloride

-
A
-
884-09-3
phenyl thiobenzoate

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In diethyl ether for 1h; | A 99% B 1% |

-
-
52867-19-3
diphenyl-N-(p-toluenesulfonyl)selenimide

-
-
108-98-5
thiophenol

-
A
-
1132-39-4
diphenylselenide

-
B
-
70-55-3
toluene-4-sulfonamide

-
C
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In chloroform for 0.25h; Product distribution; Ambient temperature; | A 96% B 99% C 97% |

-
-
55986-20-4
N-(p-tolylsulfonyl)dibenzylselenimide

-
-
108-98-5
thiophenol

-
A
-
1842-38-2
dibenzyl selenide

-
B
-
70-55-3
toluene-4-sulfonamide

-
C
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In chloroform for 0.166667h; Product distribution; Ambient temperature; | A 98% B 99% C 97% |

-
-
507233-69-4, 594-30-9, 28719-54-2
triphenyl bismuth (2+); dichloride

-
-
108-98-5
thiophenol

-
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 60℃; for 18h; | 99% |
-
-
57340-80-4
thallium thiophenoxide

-
-
75-36-5
acetyl chloride

-
A
-
934-87-2
S-phenyl thioacetate

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.5h; | A 99% B 1% |


-
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With sulfur In N,N-dimethyl-formamide at 130℃; for 5.5h; Substitution; | 99% |

-
A
-
92-52-4
biphenyl

-
B
-
139-66-2
diphenyl sulfide

-
C
-
603-36-1
triphenylantimony

-
D
-
882-33-7
diphenyldisulfane

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In neat (no solvent) thermal decompn. of Sb compd. in a sealed tube on heating to 140°C for 3 h; not isolated, detected by GLC and TLC; | A 21% B 69% C 99% D 30% E 6% |

-
-
573-17-1
9-bromophenanthrene

-
-
56741-04-9
S-phenyl tert-butylcarbamothioate

-
A
-
1579273-93-0
N-(tert-butyl)phenanthrene-9-carboxamide

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| Stage #1: 9-bromophenanthrene With iodine; magnesium In 2-methyltetrahydrofuran at 60℃; for 1h; Schlenk technique; Inert atmosphere; Stage #2: S-phenyl tert-butylcarbamothioate In 2-methyltetrahydrofuran at 0 - 20℃; for 2h; Inert atmosphere; Schlenk technique; Stage #3: With ammonium hydroxide; oxygen In 2-methyltetrahydrofuran; water under 760.051 Torr; for 18h; | A 76% B 99% |

-
-
21524-34-5
1-Bromo-2,4,6-triisopropylbenzene

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,6-triisopropyl-1-bromobenzene With iodine; magnesium In 2-methyltetrahydrofuran at 60℃; for 1h; Schlenk technique; Inert atmosphere; Stage #2: S-phenyl (1-adamantyl)thiocarbamate In 2-methyltetrahydrofuran at 0 - 20℃; for 2h; Inert atmosphere; Schlenk technique; Stage #3: With ammonium hydroxide; oxygen In 2-methyltetrahydrofuran; water under 760.051 Torr; for 18h; Schlenk technique; | A 98% B 99% |

-
-
3972-65-4
1-bromo-4-tert-butylbenzene

-
A
-
331434-35-6
N-(1-adamantyl)-4-(tert-butyl)benzamide

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-tert-butylbenzene With iodine; magnesium In 2-methyltetrahydrofuran at 60℃; for 1h; Schlenk technique; Inert atmosphere; Stage #2: S-phenyl (1-adamantyl)thiocarbamate In 2-methyltetrahydrofuran at 0 - 20℃; for 2h; Inert atmosphere; Schlenk technique; Stage #3: With ammonium hydroxide; oxygen In 2-methyltetrahydrofuran; water under 760.051 Torr; for 18h; Schlenk technique; | A 86% B 99% |


| Conditions | Yield |
|---|---|
| With iodine; triphenylphosphine In water at 100℃; Green chemistry; | 98% |
| With tungsten(VI) chloride; sodium iodide In acetonitrile for 12h; Ambient temperature; | 95% |
| With Silphos; iodine In acetonitrile for 12h; Heating; | 95% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
57340-80-4
thallium thiophenoxide

-
A
-
4551-15-9
phenylthiotrimethylsilane

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In diethyl ether for 18h; | A 98% B 2% |


| Conditions | Yield |
|---|---|
| With potassium 5-methyl-1,3,4-oxadiazole-2-thiolate; basolite C300 In water; N,N-dimethyl-formamide at 130℃; for 6h; Green chemistry; | 98% |
| With sulfur; potassium hydroxide In water at 130℃; for 7.5h; Green chemistry; | 95% |
| With copper(l) iodide; hexachloroethane; sodium carbonate; thiourea In water at 120℃; for 15h; | 93% |
-
-
87577-90-0
2-[(1-phenylmethyl)sulfinyl]pyridine

-
A
-
101-82-6
2-Benzylpyridine

-
B
-
1212-08-4
S-Phenyl benzenethiosulfonate

-
C
-
1208-20-4, 6930-77-4, 133670-27-6
S-phenyl benzenethiosulfinate

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Product distribution; Mechanism; Ambient temperature; investigation of reaction with various Grignard reagents and n-BuLi; | A 98% B 3% C 60% D 16% |
| In tetrahydrofuran Ambient temperature; | A 98% B 3% C 60% D 16% |

-
-
87577-90-0
2-[(1-phenylmethyl)sulfinyl]pyridine

-
A
-
101-82-6
2-Benzylpyridine

-
B
-
1212-08-4
S-Phenyl benzenethiosulfonate

-
C
-
1208-20-4, 6930-77-4, 133670-27-6
S-phenyl benzenethiosulfinate

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With phenylmagnesium bromide In tetrahydrofuran Ambient temperature; | A 98% B 3% C 60% D 16% |
| With phenylmagnesium bromide In tetrahydrofuran Ambient temperature; | A 98% B 3% C 60% D 16% |

-
-
506-96-7
Acetyl bromide

-
-
57340-80-4
thallium thiophenoxide

-
A
-
934-87-2
S-phenyl thioacetate

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In diethyl ether for 1h; | A 98% B 1% |

-
-
90-15-3
α-naphthol

-
-
64168-52-1
4'-nitrobenzenesulfenanilide

-
A
-
68143-73-7
2-phenylthio-1-naphthol

-
B
-
19133-53-0
4-(phenylthio)naphthalen-1-ol

-
C
-
100-01-6
4-nitro-aniline

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene for 0.166667h; Ambient temperature; Further byproducts given; | A 42% B 33% C 98% D 14% |

-
-
90-15-3
α-naphthol

-
-
64168-52-1
4'-nitrobenzenesulfenanilide

-
A
-
68143-73-7
2-phenylthio-1-naphthol

-
B
-
19133-53-0
4-(phenylthio)naphthalen-1-ol

-
C
-
100-01-6
4-nitro-aniline

-
D
-
103929-98-2
2,4-bis(phenylthio)naphthalen-1-ol

-
E
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene for 0.166667h; Product distribution; Mechanism; 1.5 eqiv. TFA; | A 42% B 33% C 98% D 10% E 14% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; methanol for 2h; Heating; | 100% |
| With sodium tetrahydroborate In tetrahydrofuran; methanol for 2h; Heating; solvent effect (t-BuOH, DME, 1,4-dioxane, EtOH); | 100% |
| With sodium diethylpiperidinohydroaluminate In tetrahydrofuran at 0℃; for 3h; | 98% |

| Conditions | Yield |
|---|---|
| With chlorine In dichloromethane at 0℃; for 0.333333h; | 100% |
| With (Dichloroiodo)benzene In dichloromethane at 20℃; for 0.333333h; | 95% |
| With chlorine In tetrachloromethane at -78℃; | 93% |

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; acetic acid at -40℃; for 3h; | 100% |
| With sulfuryl dichloride; acetic acid at -20 - 35℃; for 5.3h; | 85% |
| With sulfuryl dichloride; acetic acid at -20 - 35℃; for 6h; | 85% |

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; antimonypentachloride In 1,2-dichloro-ethane for 3h; Heating; | 100% |
| With dipotassium peroxodisulfate In trifluoroacetic acid at 20℃; for 16h; | 89% |
| Stage #1: diphenyldisulfane With thionyl chloride In 1,2-dichloro-ethane at 5 - 30℃; for 4h; Stage #2: With aluminum (III) chloride In 1,2-dichloro-ethane at 5 - 10℃; for 1h; Stage #3: methoxybenzene In 1,2-dichloro-ethane for 4h; | 88.5% |
| With silver hexafluoroantimonate; antimonypentachloride In 1,2-dichloro-ethane for 3h; Product distribution; Heating; effect of Lewis acid and silver salt; |
-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
-
882-33-7
diphenyldisulfane

-
-
2486-09-1
2,4-dinitro-1-phenylsulfanyl-benzene

| Conditions | Yield |
|---|---|
| With borax; N-Acetylcysteine In methanol; water for 1h; Ambient temperature; | 100% |
| With sodium hydroxide; Aminoiminomethanesulfinic acid; cetyltrimethylammonim bromide In tetrahydrofuran; water for 4h; Product distribution; Heating; | 95% |
| With sodium hydroxide; cetyltrimethylammonim bromide; Aminoiminomethanesulfinic acid In tetrahydrofuran for 4h; Heating; | 95% |
| With sodium hydroxide; Aminoiminomethanesulfinic acid; cetyltrimethylammonim bromide In tetrahydrofuran; water for 4h; Heating; | 95% |
| With aluminium trichloride; zinc In water; N,N-dimethyl-formamide at 65℃; for 6h; | 95% |

| Conditions | Yield |
|---|---|
| at 230 - 270℃; for 8h; Mechanism; other aryl iodides; | 100% |
| at 230 - 270℃; for 8h; | 100% |
| With caesium carbonate In dimethyl sulfoxide at 100℃; for 24h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With tributylphosphine In tetrahydrofuran at 62℃; under 7500600 Torr; for 3h; other pressures and times; var. prim. and sec. alcohols; | 100% |
| With tributylphosphine In tetrahydrofuran at 62℃; under 7500600 Torr; for 3h; | 100% |
-
-
578-58-5
2-methylmethoxybenzene

-
-
882-33-7
diphenyldisulfane

-
-
107777-12-8
1-methoxy-2-methyl-4-(phenylsulfanyl)benzene

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; antimonypentachloride In 1,2-dichloro-ethane for 3h; Heating; | 100% |
-
-
72657-23-9
methyl (R)-3-hydroxy-2-methylpropionate

-
-
882-33-7
diphenyldisulfane

-
-
121783-48-0
(S)-2-methyl-3-(phenylsulfanyl)propanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With tributylphosphine In acetonitrile for 4.5h; Ambient temperature; | 100% |
| With tributylphosphine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 95% |

| Conditions | Yield |
|---|---|
| In benzene at 35 - 45℃; for 4h; Irradiation; | 100% |
-
-
36525-02-7, 79178-05-5
<(E)-3,3-dimethyl-1-butenyl>mercuric chloride

-
-
882-33-7
diphenyldisulfane

-
-
53847-74-8
E-3,3-Dimethylbut-1-en-1-yl-phenylsulfid

| Conditions | Yield |
|---|---|
| In benzene at 35 - 45℃; for 6h; Irradiation; | 100% |
-
-
138814-74-1
2-[1,3]Dioxolan-2-ylmethyl-3,3-dimethyl-butan-1-ol

-
-
882-33-7
diphenyldisulfane

-
-
138814-75-2
2-(3,3-Dimethyl-2-phenylsulfanylmethyl-butyl)-[1,3]dioxolane

| Conditions | Yield |
|---|---|
| With pyridine; tributylphosphine at 25℃; | 100% |
-
-
87817-91-2
5-Fluoro-1-((3aR,4R,6R,6aR)-6-methoxymethoxymethyl-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxol-4-yl)-1H-pyrimidine-2,4-dione

-
-
882-33-7
diphenyldisulfane

-
-
87817-96-7
5-Fluoro-1-((3aR,4R,6R,6aR)-6-methoxymethoxymethyl-2,2-dimethyl-tetrahydro-furo[3,4-d][1,3]dioxol-4-yl)-6-phenylsulfanyl-1H-pyrimidine-2,4-dione

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -70℃; | 100% |
-
-
91798-92-4
2-(2-(but-3-yn-1-yl)-1,3-dioxolan-2-yl)ethan-1-ol

-
-
882-33-7
diphenyldisulfane

-
-
91798-93-5
2-(3-butynyl)-2-<2-(phenylthio)ethyl>-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With tributylphosphine In benzene for 0.5h; | 100% |
-
-
882-33-7
diphenyldisulfane

-
-
67319-06-6
1-ethoxymethyl-5-lithio-2-phenylthioimidazole

-
-
86051-84-5
1-ethoxymethyl-2,5-bis(phenylthio)imidazole

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -70℃; | 100% |
| With n-butyllithium In tetrahydrofuran at -78℃; | 100% |
-
-
882-33-7
diphenyldisulfane

-
-
4064-06-6
1,2:3,4-di-O-isopropylidene-α-D-galactopyranose

-
-
104495-07-0
1,2:3,4-di-O-isopropylidene-6-S-phenyl-6-thio-α-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With tributylphosphine In tetrahydrofuran at 62℃; under 10000 Torr; for 5h; | 100% |
| With tributylphosphine In pyridine at 20℃; for 24h; | 96% |
| With pyridine; tributylphosphine for 20h; Ambient temperature; | 95% |
| With tributylphosphine In pyridine for 12h; Ambient temperature; | 86% |
-
-
882-33-7
diphenyldisulfane

-
-
176043-45-1
(E)-5-[(2R,3R)-3-((E)-4,8-Dimethyl-nona-3,7-dienyl)-3-methyl-oxiranyl]-2-methyl-pent-2-en-1-ol

-
-
176043-46-2
(2R,3R)-2-((E)-4,8-Dimethyl-nona-3,7-dienyl)-2-methyl-3-((E)-4-methyl-5-phenylsulfanyl-pent-3-enyl)-oxirane

| Conditions | Yield |
|---|---|
| With tributylphosphine In dichloromethane for 0.5h; Ambient temperature; | 100% |
-
-
882-33-7
diphenyldisulfane

-
-
181576-34-1
(Z)-6-(1-Methylcyclohexa-2,5-dienyl)hex-5-en-1-ol

-
-
252869-48-0
3-Methyl-3-[(Z)-6-(phenylsulfenyl)hex-1-enyl]cyclohexa-1,4-diene

| Conditions | Yield |
|---|---|
| With pyridine; tributylphosphine | 100% |
| With tributylphosphine In pyridine at 20℃; for 7h; Substitution; | 100% |
-
-
80657-57-4
3-hydroxy-(2S)-methylpropionate

-
-
882-33-7
diphenyldisulfane

-
-
165457-66-9
(R)-2-methyl-3-(phenylsulfanyl)propanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With tributylphosphine In tetrahydrofuran at 20℃; for 24h; | 100% |
| With tributylphosphine In tetrahydrofuran for 24h; Ambient temperature; | 99% |
| With tributylphosphine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 85% |
| With tributylphosphine In N,N-dimethyl-formamide at 0 - 25℃; for 5h; Yield given; |
-
-
882-33-7
diphenyldisulfane

-
-
248256-35-1
(1S,2R,3R)-1-acetoxymethyl-2-hydroxymethyl-3-phenylcyclopropane

-
-
392656-02-9
Acetic acid (1S,2S,3R)-2-phenyl-3-phenylsulfanylmethyl-cyclopropylmethyl ester

| Conditions | Yield |
|---|---|
| With tributylphosphine In pyridine at 20℃; for 1h; | 100% |
| With tributylphosphine In pyridine at 20℃; Substitution; |
-
-
90125-52-3, 90125-53-4, 107796-93-0, 107796-94-1, 121651-18-1, 147513-36-8
(+/-)-(2R,3R)-2,3-epoxy-1-nonanol

-
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With tributylphosphine In pyridine at 0℃; | 100% |
-
-
882-33-7
diphenyldisulfane

-
-
392655-66-2
(2S)-2-vinyl-3-phenyl-1-propanol

| Conditions | Yield |
|---|---|
| With tributylphosphine In pyridine at 20℃; for 1h; | 100% |
-
-
882-33-7
diphenyldisulfane


-
-
474248-58-3
[(2S,3R,5S,6R)-5-Benzyloxy-6-benzyloxymethyl-2-((R)-1-benzyloxy-3-phenylsulfanyl-propyl)-2-methyl-tetrahydro-pyran-3-yloxy]-triisopropyl-silane

| Conditions | Yield |
|---|---|
| With tributylphosphine In pyridine at 20℃; for 24h; | 100% |
-
-
34341-05-4
cyclopentadecanone oxime

-
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With tributylphosphine In tetrahydrofuran at 20℃; | 100% |
Diphenyl disulfide Chemical Properties
The Molecular formula of PHENYL DISULFIDE(882-33-7):C12H10S2
The Molecular Weight of PHENYL DISULFIDE(882-33-7):218.34
The Molecular Structure of PHENYL DISULFIDE(882-33-7) is: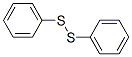
Density:1.22 g/cm3
Melting point:58-60 °C
Boiling point:310 °C at 760 mmHg
Flash point:177.8 °C
Index of Refraction:1.679
FEMA:3225
BRN:639794
Molar Refractivity:67.49 cm3
Molar Volume:178.6 cm3
Polarizability:26.75 10-24cm3
Surface Tension:53.1 dyne/cm
Enthalpy of Vaporization:52.88 kJ/mol
Vapour Pressure:0.00113 mmHg at 25°C
Appearance:white to light yellow crystal.
IUPAC Name:phenyldisulfanylbenzene
Synonyms:PHENYL DISULFIDE;BIPHENYL DISULFIDE;PHENYL DISULPHIDE;DIPHENYL DISULFIDE;DIPHENYL DISULPHIDE;DIPHENYLDISULPIDE;FEMA 3225;AKOS 94361
Diphenyl disulfide Uses
PHENYL DISULFIDE with its cas registry number(882-33-7) could be used as a pesticide, medicine, dyes intermediates.
Diphenyl disulfide Production
Ph2S2 is usually prepared by the oxidation of thiophenol:
2PhSH + I2 → Ph2S2 + 2HI
Hydrogen peroxide can also be used as the oxidant.Ph2S2 is rarely prepared in the laboratory because it is inexpensive, and the precursor has a disagreeable odour.
Like most organic disulfides, the C2S2 core of Ph2S2 is non-planar with a dihedral angle approaching 85°.
Diphenyl disulfide Toxicity Data With Reference
| 1. | ipr-mus LD50:100 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . |
Diphenyl disulfide Consensus Reports
Reported in EPA TSCA Inventory.
Diphenyl disulfide Safety Profile
Poison by intraperitoneal route. When heated to decomposition it emits toxic fumes of SOx. Flammable when exposed to heat or flame. See also SULFIDES.
Hazard Codes:Xi
Xi:Irritant
Risk Statements
R36/37/38:Irritating to eyes, respiratory system and skin .
Safety Statements
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S36:Wear suitable protective clothing .
RIDADR:UN 3335
WGK Germany :3
RTECS:SS6825000
F:13
Hazard Note:Irritant
TSCA:T
Diphenyl disulfide Specification
Chemical Stability: Stable.
Incompatibilities with Other Materials Strong oxidizing agents, strong reducing agents, strong bases.
Hazardous Polymerization Will not occur.
Hazardous Decomposition Products Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide, hydrogen sulfide.
Conditions to Avoid: Incompatible materials.
Related Products
- Diphenyl carbonate
- Diphenyl chlorophosphate
- Diphenyl diselenide
- Diphenyl disulfide
- Diphenyl ether
- Diphenyl isononyl phosphinate
- Diphenyl isophthalate
- Diphenyl N-cyanocarbonimidate
- Diphenyl phosphate
- Diphenyl Phosphite
- 88235-25-0
- 882-36-0
- 88239-63-8
- 882402-12-2
- 882423-68-9
- 882430-69-5
- 88-24-4
- 88247-84-1
- 88247-87-4
- 882499-87-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View