-
Name
3-Hydroxytyramine
- EINECS 200-110-0
- CAS No. 51-61-6
- Article Data59
- CAS DataBase
- Density 1.248 g/cm3
- Solubility
- Melting Point 218-220 oC
- Formula C8H11NO2
- Boiling Point 337.69 °C at 760 mmHg
- Molecular Weight 153.181
- Flash Point 158.03 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Pyrocatechol,4-(2-aminoethyl)- (8CI);2-(3,4-Dihydroxyphenyl)-1-ethanamine;2-(3,4-Dihydroxyphenyl)ethylamine;3,4-Dihydroxyphenylethylamine;3-Hydroxytyramine;4-(2-Aminoethyl)-1,2-benzenediol;4-(2-Aminoethyl)catechol;Hydroxytyramin;NSC 173182;Oxytyramine;
- PSA 66.48000
- LogP 1.29930
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; aromatic L-amino acid decarboxylase In various solvent(s) at 30℃; for 48h; | 82% |
| beim Erhitzen ueber den Schmelzpunkt; | |
| With NH4OH-NH4Cl buffer; pyridoxal 5'-phosphate at 30℃; for 0.5h; relative rate of CO2 evolution by aromatic L-amino acid decarboxylase from Micrococcus percitreus; |

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) perchlorate; ascorbic acid In water | 19% |
| With P4502D6 Kinetics; Enzymatic reaction; |
-
-
81666-88-8
3-amino-4-hydroxyphenylethylamine

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With barium nitrite; sulfuric acid Diazotization.und Eingiessen der Loesung in siedende Kupfersulfat-Loesung; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 170℃; | |
| With hydrogen bromide at 130℃; | |
| With hydrogenchloride at 150℃; |
-
-
525-72-4, 27740-96-1, 53622-83-6, 78579-88-1
(+/-)-salsolinol

-
A
-
51-61-6
dopamine

-
B
-
53405-13-3, 102917-28-2
1,2,3,4-tetrahdyro-1-methylisopquinoline-7,8-diol

| Conditions | Yield |
|---|---|
| In water at 180℃; for 28h; Product distribution; var. time; |
-
-
57894-18-5
2-[2-(3,4-dihydroxyphenyl)ethyl]-1,3-dioxoisoindoline

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With hydrazine |
-
-
37034-31-4
N-(tert-butoxycarbonyl)dopamine

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With methoxybenzene; trifluoroacetic acid | |
| Multi-step reaction with 3 steps 1: caesium carbonate / acetone / 24 h / 20 °C 2: chloro-trimethyl-silane / methanol / 20 °C 3: aq. buffer / pH 7.2 / Irradiation View Scheme |
-
-
37034-22-3
N-benzyloxycarbonyl-3,4-dihydroxyphenylethylamine

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal |

| Conditions | Yield |
|---|---|
| In water at 180℃; for 66h; Mechanism; other tetraisoquinolines; |
-
-
76734-99-1
2-(3',4'-dihydroxyphenyl)-3-acetylamino-6-(N-acetyl-2''-aminoethyl)-2,3-dihydro-1,4-benzodioxin

-
A
-
51-61-6
dopamine

-
B
-
499-61-6
arterenone

-
C
-
29477-54-1
2-hydroxy-3′,4′-dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 110℃; for 3h; |

-
-
76734-99-1
2-(3',4'-dihydroxyphenyl)-3-acetylamino-6-(N-acetyl-2''-aminoethyl)-2,3-dihydro-1,4-benzodioxin

-
A
-
51-61-6
dopamine

-
B
-
29477-54-1
2-hydroxy-3′,4′-dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 3h; Heating; |
-
-
619-60-3
4-(N,N-dimethylamino)phenol

-
A
-
51-61-6
dopamine

-
B
-
54737-34-7
4-(N,N-dimethylamino)phenoxyl radical

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water Equilibrium constant; Irradiation; |

-
-
50673-96-6
dopaminoquinone

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| Rate constant; pH 7.00; reaction with substrate reduced glucose oxidase; | |
| With citric acid In phosphate buffer pH=6.3; | |
| With sulfuric acid In water at 22℃; pH=1; Electrolysis; Inert atmosphere; |

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| ueber den Schmelzpunkt; |

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; platinum Hydrogenation; |

| Conditions | Yield |
|---|---|
| With hydrogen iodide |

| Conditions | Yield |
|---|---|
| Reaktion des Hydrochlorids; |


-
-
51-61-6
dopamine

-
-
51-61-6
dopamine

-
-
51-61-6
dopamine

-
-
51-61-6
dopamine

-
-
51-61-6
dopamine

-
-
51-61-6
dopamine

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iron(II) sulfate |

| Conditions | Yield |
|---|---|
| und Eintragen der Diazoloesung in siedende konzentrierte Kupfersulfat-Loesung; |


| Conditions | Yield |
|---|---|
| With tyrosinase In phosphate buffer for 0.333333h; pH=6.3; |
-
-
37627-79-5
N-[2-(3-methoxy-4-hydroxy-phenyl)ethyl]phthalimide

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 14 percent / oxygen, cupric perchlorate, ascorbic acid / H2O; acetone / 24 h / 60 °C 2: NH2NH2 View Scheme |
-
-
23699-77-6
tert-butyl 4-hydroxy-3-methoxyphenethylcarbamate

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 28 percent / oxygen, cupric perchlorate, ascorbic acid, monosodium ascorbate / H2O; acetone / 27 h / 20 °C 2: TFA, PhOMe View Scheme |
-
-
51-61-6
dopamine

-
-
21581-49-7
4-(2-aminoethyl)-5-nitrobenzene-1,2-diol

| Conditions | Yield |
|---|---|
| With phosphate buffer pH 7.4; sodium nitrite Ambient temperature; | 100% |
-
-
51-61-6
dopamine

-
-
7339-87-9
4-hydroxyphenylacetaldehyde

-
-
5843-65-2, 17072-47-8, 106032-53-5, 22672-77-1
(1S)-1-(4-hydroxybenzyl)-1,2,3,4-tetrahydroisoquinoline-6,7-diol

| Conditions | Yield |
|---|---|
| With CjNCS2 In acetonitrile at 37℃; for 3h; pH=7.4; Enzymatic reaction; | 99% |
| With recombinant Thalictrum flavum norcoclaurine synthase; 2-amino-2-hydroxymethyl-1,3-propanediol In methanol; water at 20℃; for 3h; pH=7; Pictet-Spengler cyclisation; Enzymatic reaction; enantioselective reaction; | |
| With Norcoclaurine synthase Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 5h; Cooling with ice; | 97% |
-
-
51-61-6
dopamine

-
-
75-87-6
chloral

-
-
115710-37-7
1-trichloromethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid Heating; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: dopamine; D-(+)-ribonic acid gamma-lactone In methanol for 0.166667h; Stage #2: With triethylamine In methanol for 4h; Reflux; Darkness; | 94.1% |
-
-
51-61-6
dopamine

-
-
10199-34-5, 14056-88-3, 15604-36-1
cis-dichlorobis(triphenylphosphine)platinum(II)

| Conditions | Yield |
|---|---|
| With potassium hydroxide; dichloromethane In methanol; benzene to a suspn. of substituted catechol in benzene was added MeOH soln. of KOH; prepd. soln. was syringed into suspn. of Pt complex in benzene; stirring at room temp. for 3.5 h (Ar); filtration, evapn., washing with water, drying in vac., dissoln. in CH2Cl2, filtration, evapn., washing with ether, drying in vac.; elem. anal.; | 94% |
-
-
51-61-6
dopamine

-
-
75991-86-5
demethyldihydrocorynantheine

| Conditions | Yield |
|---|---|
| In methanol for 4h; Ambient temperature; | 93% |
-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium nitrite | 93% |

| Conditions | Yield |
|---|---|
| With pyridine; 1,1,1,3',3',3'-hexafluoro-propanol for 8h; Pictet-Spengler Synthesis; Reflux; Inert atmosphere; | 93% |
-
-
51-61-6
dopamine

-
-
66753-05-7
<5,6,8,9,11,12,14,15-3H8>arachidonic acid

| Conditions | Yield |
|---|---|
| Stage #1: <5,6,8,9,11,12,14,15-3H8>arachidonic acid With triethylamine; isobutyl chloroformate In acetonitrile at 23℃; for 2h; Stage #2: dopamine In N,N-dimethyl-formamide at 23℃; for 20h; | 91% |
-
-
51-61-6
dopamine

-
-
21814-48-2
1-chloro-7-methoxy-4-nitro-10H-acridin-9-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1,4-dioxane for 9h; Heating; | 90.1% |

| Conditions | Yield |
|---|---|
| With Halomonas elongata/Co imm pyridoxal phosphate In toluene at 37℃; for 0.25h; pH=7.5; Flow reactor; Enzymatic reaction; | 90% |
| With monoamine oxidases | |
| With transaminase from chromobacterium violaceum; sodium pyruvate Enzymatic reaction; |
-
-
51-61-6
dopamine

-
-
85-44-9
phthalic anhydride

-
-
57894-18-5
2-[2-(3,4-dihydroxyphenyl)ethyl]-1,3-dioxoisoindoline

| Conditions | Yield |
|---|---|
| at 100 - 150℃; Green chemistry; | 90% |
-
-
51-61-6
dopamine

-
-
64-18-6
formic acid

-
-
75-87-6
chloral

-
-
115684-30-5
6,7-Dihydroxy-1-trichloromethyl-3,4-dihydro-1H-isoquinoline-2-carbaldehyde

| Conditions | Yield |
|---|---|
| Heating; | 89% |


| Conditions | Yield |
|---|---|
| Stage #1: 6-mercaptocaproic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide for 0.25h; Stage #2: dopamine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide for 3h; | 89% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 12h; Cooling with ice; | 86% |


| Conditions | Yield |
|---|---|
| With TEA In methanol for 0.5h; | 85.5% |
| With sodium hydroxide; potassium hydrogensulfate In 1,4-dioxane; water; ethyl acetate | 2.6 g (78%) |

| Conditions | Yield |
|---|---|
| at 110℃; for 1h; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 5 - 40℃; for 2h; | 85% |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
51-61-6
dopamine

| Conditions | Yield |
|---|---|
| In methanol for 24h; Reagent/catalyst; Reflux; | 83% |
-
-
51-61-6
dopamine

-
-
501-53-1
benzyl chloroformate

-
-
37034-22-3
N-benzyloxycarbonyl-3,4-dihydroxyphenylethylamine

| Conditions | Yield |
|---|---|
| With sodium carbonate In diethyl ether; water at 0 - 20℃; for 3h; | 82% |
| With diethyl ether; sodium hydrogencarbonate | |
| With sodium hydroxide In water; toluene at 5 - 10℃; for 2h; |
-
-
51-61-6
dopamine

-
-
23911-26-4
diethylenetriaminepentaacetic dianhydride

-
-
147666-97-5
N,N'-bis(3-hydroxytyramide)diethylenetriamine N,N',N''-triacetic Acid

| Conditions | Yield |
|---|---|
| With ascorbic acid In N,N-dimethyl-formamide at 60℃; | 79% |

| Conditions | Yield |
|---|---|
| With pyridine for 6h; | 78% |

| Conditions | Yield |
|---|---|
| With pyridine for 6h; | 78% |
-
-
51-61-6
dopamine

-
-
60-33-3
linoleic acid

-
-
105955-12-2
(Z,Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]-octadeca-9,12-dienamide

| Conditions | Yield |
|---|---|
| Stage #1: linoleic acid With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 0.5h; Stage #2: dopamine In dichloromethane for 12h; | 77% |
| Stage #1: linoleic acid With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In ethyl acetate at 20℃; for 1h; Stage #2: dopamine In ethyl acetate at 20℃; for 12h; |
-
-
51-61-6
dopamine

-
-
28920-43-6
(fluorenylmethoxy)carbonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: dopamine With sodium hydrogencarbonate In water at 0℃; for 0.25h; Stage #2: (fluorenylmethoxy)carbonyl chloride In water; acetonitrile at 0 - 20℃; for 17.5h; | 77% |
| Stage #1: dopamine With sodium hydrogencarbonate In water at 0℃; for 0.25h; Stage #2: (fluorenylmethoxy)carbonyl chloride In acetonitrile at 0 - 20℃; for 17.5h; | 77% |
| In water at 60℃; for 4h; chemoselective reaction; | 75% |
-
-
51-61-6
dopamine

-
-
27200-79-9
p-bromophenylacetaldehyde

-
-
1421820-24-7
(1S)-1-(4-bromobenzyl)-1,2,3,4-tetrahydroisoquinoline-6,7-diol

| Conditions | Yield |
|---|---|
| With CjNCS2 In acetonitrile at 37℃; for 3h; pH=7.4; Enzymatic reaction; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-diphenylphosphanobenzoic acid With N-Bromosuccinimide; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; for 2h; Stage #2: dopamine With sodium hydrogencarbonate In water; N,N-dimethyl-formamide at 25℃; | 76.8% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; acetic acid In methanol Molecular sieve; Dean-Stark; Reflux; | 76% |
Dopamine History
The function of Dopamine (CAS NO.51-61-6) as a neurotransmitter was discovered in 1958 by Arvid Carlsson and Nils-?ke Hillarp at the Laboratory for Chemical Pharmacology of the National Heart Institute of Sweden. It was named dopamine(51-61-6) because it was a monoamine, and its synthetic precursor was 3,4-dihydroxyphenylalanine (L-DOPA). Arvid Carlsson was awarded the 2000 Nobel Prize in Physiology or Medicine for showing that dopamine(51-61-6) is not just a precursor of norepinephrine (noradrenaline) and epinephrine (adrenaline) but a neurotransmitter, as well.
Dopamine(51-61-6) was first synthesized in 1910 by George Barger and James Ewens at Wellcome Laboratories in London, England.
Dopamine Specification
The CAS registry number of Dopamine is 51-61-6. In addition, the molecular formula is C8H11NO2. It is a catecholamine neurotransmitter which present in a wide variety of animals, including both vertebrates and invertebrates. And it is produced in several areas of the brain, including the substantia nigra and the ventral tegmental area. Moreover, its main function as a hormone is to inhibit the release of prolactin from the anterior lobe of the pituitary.
Physical properties about this chemical are: (1)ACD/LogP: 0.05; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 1; (5)ACD/KOC (pH 7.4): 1; (6)#H bond acceptors: 3; (7)#H bond donors: 4; (8)#Freely Rotating Bonds: 5; (9)Polar Surface Area: 66.48 Å2; (10)Index of Refraction: 1.619; (11)Molar Refractivity: 43.101 cm3; (12)Molar Volume: 122.788 cm3; (13)Polarizability: 17.086 ×10-24cm3; (14)Surface Tension: 60.841 dyne/cm; (15)Density: 1.248 g/cm3; (16)Flash Point: 158.03 °C; (17)Enthalpy of Vaporization: 60.399 kJ/mol; (18)Boiling Point: 337.69 °C at 760 mmHg.
Preparation of Dopamine: it is synthesized by demethylation of 2-(3,4-dimethoxyphenyl)ethylamine through using hydrogen bromide.

Uses of Dopamine: it can be used as a medicinal agent. And it is available as an intravenous medication acting on the sympathetic nervous system, and can increase heart rate and blood pressure. In addition, it can react with N-Methoxydiacetamide to get N-(3,4-dihydroxy-phenethyl)-acetamide. This reaction will need reagent sodium acetate and solvent dimethylformamide. The reaction time is 4.5 hours with ambient temperature. The yield is about 31%.
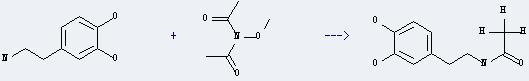
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(c(cc1CCN)O)O
(2)InChI: InChI=1/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2
(3)InChIKey: VYFYYTLLBUKUHU-UHFFFAOYAA
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | unreported | 1120mg/kg (1120mg/kg) | Khimiko-Farmatsevticheskii Zhurnal. Chemical Pharmaceutical Journal. For English translation, see PCJOAU. Vol. 24(4), Pg. 42, 1990. | |
| mouse | LD50 | intracervical | 74mg/kg (74mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Tohoku Yakka Daigaku Kenkyu Nempo. Annual Report of the Tohoku College of Pharmacy. Vol. 27, Pg. 131, 1980. |
| mouse | LD50 | intraperitoneal | 950mg/kg (950mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION BLOOD: HEMORRHAGE | Oyo Yakuri. Pharmacometrics. Vol. 8, Pg. 835, 1974. |
| mouse | LD50 | intravenous | 59mg/kg (59mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER CHANGES: OLFACTION LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION BLOOD: HEMORRHAGE | Oyo Yakuri. Pharmacometrics. Vol. 8, Pg. 835, 1974. |
| rat | LD50 | intraperitoneal | 163mg/kg (163mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Toxicology and Applied Pharmacology. Vol. 88, Pg. 433, 1987. |
Related Products
- Dopamine
- Dopamine hydrochloride
- 51618-29-2
- 51618-30-5
- 51619-55-7
- 51619-56-8
- 5162-03-8
- 51622-32-3
- 5162-44-7
- 51627-14-6
- 51627-47-5
- 51628-12-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View