-
Name
Ethoxytrimethylsilane
- EINECS 217-370-6
- CAS No. 1825-62-3
- Article Data146
- CAS DataBase
- Density 0.772 g/cm3
- Solubility reacts with water
- Melting Point -83 °C
- Formula C5H14OSi
- Boiling Point 75.5 °C at 760 mmHg
- Molecular Weight 118.251
- Flash Point -18 °C
- Transport Information UN 1993 3/PG 2
- Appearance clear colorless liquid
- Safety 16-26-9-37/39-33
- Risk Codes 11-22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn,
Xn,  Xi
Xi
- Synonyms Ethyl trimethylsilyl ether;Trimethylsilicon ethoxide;Trimethylsilyl ethoxide;Trimethylsilyl ethyl ether;LS 875;NSC 43345;Silane M 3-ethoxy;Trimethylethoxysilane;
- PSA 9.23000
- LogP 1.85780
Synthetic route

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 27℃; for 3h; | 100% |
-
-
64-17-5
ethanol

-
-
114518-51-3
N,N'-bis<(trimethylsiloxy)carbonyl>-N,N'-bis(trimethylsilyl)hydrazine

-
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| 97.5% |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium phthalimide-N-oxyl In ethyl acetate for 1h; Reflux; | 96% |
| With [nano-MCM-41(at)(CH2)3-1-methylimidazole]Br3 In dichloromethane at 20℃; for 0.416667h; | 90% |
-
-
122-51-0
orthoformic acid triethyl ester

-
A
-
74-96-4
ethyl bromide

-
B
-
1825-62-3
ethyl trimethylsilyl ether

-
C
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; sodium bromide for 2h; Product distribution; Heating; Me3SiBr generated in situ; | A n/a B n/a C 92% |

-
-
64-17-5
ethanol

-
-
17881-28-6
N,N'-trimethylsilyl(phenyl)diazene

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
1227476-15-4
Azobenzene

-
C
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| for 0.5h; Ambient temperature; | A 92% B 0.01 mmol C 66% |


| Conditions | Yield |
|---|---|
| chloro-trimethyl-silane at 20℃; for 0.0166667h; | 90% |
-
-
5573-62-6
(ethylthio)trimethylsilane

-
-
122-51-0
orthoformic acid triethyl ester

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
6267-24-9
tris(ethylsulfanyl)methane

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In toluene at 0℃; for 5h; | A 85% B 90% |

-
-
122-51-0
orthoformic acid triethyl ester

-
-
153080-50-3
C7H19PS4Si

-
A
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| at 20℃; for 2h; | A 90% B 69% |

-
-
64-17-5
ethanol

-
-
56002-69-8
trimethyl-(1H,1H,3H-tetrafluoropropoxy)silane

-
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| at 20℃; for 1h; | 88.9% |
-
-
78-08-0
Triethoxyvinylsilane

-
-
77214-46-1
N-<(β-trimethylsiloxy)ethyl>formamide

-
A
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide for 0.166667h; Yields of byproduct given; | A n/a B 86% |

-
-
7352-03-6
N-(ethoxymethyl)diethylamine

-
-
145861-40-1
2-<2-(trimethylsiloxy)ethoxy>-1,3,2-dioxaphospholane

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
128429-58-3
5-<(diethylamino)methyl>-1,4,6,9-tetraoxa-5λ5-phosphaspiro<4.4>nonane

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 120℃; | A 86% B 58% |
| With zinc(II) chloride | A n/a B 58% |
| With zinc(II) chloride at 130℃; Inert atmosphere; | A n/a B 58% |

-
-
75-77-4
chloro-trimethyl-silane

-
-
818-38-2
Diethyl glutarate

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
6838-66-0
1,2-bis((trimethylsilyl)oxy)cyclopent-1-ene

| Conditions | Yield |
|---|---|
| With benzophenone; sodium In toluene at 50℃; Concentration; Inert atmosphere; | A Ca. 70.9 g B 83.5% |


| Conditions | Yield |
|---|---|
| With urea at 20℃; for 4h; | 80.2% |
| With pyridine; xylene | |
| With pyridine In diethyl ether for 3h; Heating; |
-
-
64-17-5
ethanol

-
-
32115-55-2
N,N-Dimethylcarbamidsaeure-trimethylsilylester

-
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| for 0.0833333h; Ambient temperature; | 77% |
-
-
426-65-3
ethyl Pentafluoropropionate

-
-
10519-96-7
potassium trimethylsilonate

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
378-76-7
Potassium pentafluoropropionate

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 2h; Inert atmosphere; | A n/a B 77% |

-
-
344409-08-1
3-trimethylsilyl-4-trichloromethyl-2-oxetanone

-
-
64-17-5
ethanol

-
A
-
19486-93-2
ethyl 4,4,4-trichloro-3-hydroxybutyrate

-
B
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| at 130 - 150℃; for 100h; | A 76% B n/a |

-
-
106821-07-2
1-(Ethoxycarbonyl)-4-<4-(trimethylsilyloxy)phenyl>semicarbazid

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
106821-08-3
4-(4-Hydroxyphenyl)-1,2,4-triazolidin-3,5-dion

| Conditions | Yield |
|---|---|
| at 215℃; Yields of byproduct given; | A n/a B 75% |

| Conditions | Yield |
|---|---|
| With CH3Cl; Mg In diethyl ether | 73% |
| With methylene chloride; magnesium In diethyl ether | 73% |
| With methylene chloride; magnesium In diethyl ether introduction of CH3Cl in a suspension of Mg in a mixt. of (CH3)2Si(OC2H5)2 and ether;; | 70% |
| With methylene chloride; magnesium In diethyl ether introduction of CH3Cl in a suspension of Mg in a mixt. of (CH3)2Si(OC2H5)2 and ether;; | 70% |
-
-
64-17-5
ethanol

-
-
87651-34-1
pentachloro-2-(trimethylsiloxy)propene

-
A
-
67-66-3
chloroform

-
B
-
1825-62-3
ethyl trimethylsilyl ether

-
C
-
535-15-9
ethyl 1,1-dichloroacetate

| Conditions | Yield |
|---|---|
| With triethylamine for 8h; Heating; Yields of byproduct given; | A n/a B n/a C 71% |
| With triethylamine for 8h; Heating; Yield given; | A n/a B n/a C 71% |

-
-
86517-51-3
1-trimethylsiloxy-5-dimethylethoxysilylpentane

-
A
-
18269-45-9
1-oxa-2-sila-2,2-dimethylcycloheptane

-
B
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With sodium ethanolate 1) heating (byproduct removal), 2) heating (to distill); further catalysts; method for preparation of 1-oxo-2-sila-2,2-disubstituted cycloheptanes and cyclooctanes; | A 70% B n/a |
| With sodium ethanolate 1) heating (byproduct removal), 2) heating (to distill); Yield given. Multistep reaction; |
-
-
75-77-4
chloro-trimethyl-silane

-
-
122-51-0
orthoformic acid triethyl ester

-
-
122-52-1
triethyl phosphite

-
A
-
75-00-3
chloroethane

-
B
-
1825-62-3
ethyl trimethylsilyl ether

-
C
-
17997-33-0
diethyl(diethoxymethyl)phosphonate

| Conditions | Yield |
|---|---|
| With also with acetic anhydride instead Me3SiCl (90 -100 deg C, 5 h) Product distribution; | A n/a B n/a C 60% |

-
-
16029-98-4
trimethylsilyl iodide

-
-
1516-80-9
ethoxytriphenylsilane

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
18602-95-4
triphenylsilyl iodide

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 4h; regioselective reaction; | A n/a B 60% |

-
-
18811-63-7
N-(ethoxymethyl)morpholine

-
-
145861-40-1
2-<2-(trimethylsiloxy)ethoxy>-1,3,2-dioxaphospholane

-
A
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With zinc(II) chloride | A n/a B 57% |
| With zinc(II) chloride at 120℃; | A n/a B 52% |
| With zinc(II) chloride at 130℃; Inert atmosphere; | A n/a B 52% |


| Conditions | Yield |
|---|---|
| at 60℃; for 48h; | A n/a B 55% |

-
-
3275-13-6
1-(ethoxymethyl)piperidine

-
-
145861-40-1
2-<2-(trimethylsiloxy)ethoxy>-1,3,2-dioxaphospholane

-
A
-
1825-62-3
ethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 120℃; | A n/a B 54% |
| With zinc(II) chloride at 130℃; Inert atmosphere; | A n/a B 54% |
| With zinc(II) chloride | A n/a B 52% |

-
-
109-89-7
diethylamine

-
-
13716-45-5
diethyl trimethylsilyl phosphite

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
20262-87-7
diethyl diethylphosphoramidite

-
C
-
16141-72-3
diethyl-phosphonamidic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; | A n/a B n/a C 52% |

-
-
109-89-7
diethylamine

-
-
13716-45-5
diethyl trimethylsilyl phosphite

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
16141-72-3
diethyl-phosphonamidic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; Product distribution; Mechanism; with dibutylamine and with morpholine; | A n/a B 52% |
| at 100℃; for 1h; | A n/a B 52% |

-
-
64-17-5
ethanol

-
-
53127-77-8
acetamide O-(trimethylsilyl)oxime

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
118493-85-9, 117771-39-8, 1141922-26-0
acetamide oxime

| Conditions | Yield |
|---|---|
| at 25℃; | A 47% B 50% |

-
-
762-04-9
phosphonic acid diethyl ester

-
-
996-50-9
N,N-diethyl-1,1,1-trimethylsilanamine

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
16141-72-3
diethyl-phosphonamidic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; Product distribution; Mechanism; | A n/a B 50% |
| at 100℃; for 1h; | A n/a B 50% |

-
-
64-17-5
ethanol

-
-
770-09-2
benzyltrimethylsilane

-
A
-
1825-62-3
ethyl trimethylsilyl ether

-
B
-
539-30-0
1-(ethoxymethyl)benzene

| Conditions | Yield |
|---|---|
| With copper(II) bis(tetrafluoroborate) In acetonitrile for 10h; Irradiation; | A n/a B 48% |

-
-
1825-62-3
ethyl trimethylsilyl ether


| Conditions | Yield |
|---|---|
| Substitution; | 99% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
195319-42-7
(Cl)B(Si(CH3)3)CH((CH3)4C6H)B((CH3)4C6H)B(Si(CH3)3)CH

-
-
277742-11-7
(C2H5O)B(Si(CH3)3)CH((CH3)4C6H)B((CH3)4C6H)B(Si(CH3)3)CH

| Conditions | Yield |
|---|---|
| 99% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
7727-18-6
vanadium(V) oxychloride

-
-
1635-99-0
chloroorthovanadic acid diethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: (CH3)3SiCl; (N2); addn. of 3 equiv. of silane deriv. to CH2Cl2 soln. of vanadium compd. at room temp., stirring for 2 h at room temp.; evapn., elem. anal.; | 99% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
832076-19-4
bis(1,2,4-tri-tert-butylcyclopentadienyl)cerium hydride

-
-
1256136-75-0
[1,2,4-tri-tert-butylcyclopentadienyl]2CeOEt

| Conditions | Yield |
|---|---|
| In benzene-d6 (under inert atm., Schlenk); Ce-complex dissolved in C6D6 in NMR tube, ligand added, 2 min; monitored by NMR; | 99% |
| In pentane (under inert atm., Schlenk); Ce-complex dissolved in pentane, ligand added, 10 min, after 30 min taken to dryness, sealed under vac. in ampoule,sublimed at 185°C; elem. anal.; | 25% |

| Conditions | Yield |
|---|---|
| With iron(II) bromide at 25℃; for 0.0833333h; Reagent/catalyst; Temperature; | 99% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
123-62-6
propionic acid anhydride

-
-
16844-98-7
trimethylsilyl propionate

| Conditions | Yield |
|---|---|
| With CBV780 at 60℃; for 0.0833333h; Microwave irradiation; | 99% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
93-97-0
benzoic acid anhydride

-
-
2078-12-8
trimethylsilyl benzoate

| Conditions | Yield |
|---|---|
| With CBV780 at 120℃; for 0.0833333h; Temperature; Microwave irradiation; | 99% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
76782-91-7
(4-isopropylphenyl)dibromoborane

-
A
-
476156-89-5
4-(diethoxyboryl)cumene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | A 98% B n/a |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
76782-91-7
(4-isopropylphenyl)dibromoborane

-
-
476156-89-5
4-(diethoxyboryl)cumene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 98% |
-
-
1029505-73-4
dimethyl 5-fluoroadamantane-1,3-dicarboxylate

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
1158076-77-7
dimethyl 5-ethoxyadamantane-1,3-dicarboxylate

| Conditions | Yield |
|---|---|
| With aluminium(III) triflate In dichloromethane at 84℃; for 8h; | 98% |

| Conditions | Yield |
|---|---|
| With cesium salt nanocrystal of tungstosilicic acid In acetonitrile at 20℃; for 2.5h; Sakurai reaction; | 98% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
100-52-7
benzaldehyde

-
-
762-72-1
allyl-trimethyl-silane

-
-
54703-48-9
(1-ethoxy-but-3-enyl)benzene

| Conditions | Yield |
|---|---|
| With 35 percent H3PW12O40 modified aluminium-incorporated mesoporous KIT-6 In acetonitrile at 20℃; for 2h; Reagent/catalyst; Solvent; Temperature; Hosomi-Sakurai Reaction; Green chemistry; | 98% |
| With cesium salt nanocrystal of tungstosilicic acid In acetonitrile at 20℃; for 2h; Sakurai reaction; | 95% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
762-72-1
allyl-trimethyl-silane

| Conditions | Yield |
|---|---|
| With cesium salt nanocrystal of tungstosilicic acid In acetonitrile at 20℃; for 3h; Sakurai reaction; | 98% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
762-72-1
allyl-trimethyl-silane

| Conditions | Yield |
|---|---|
| With 35 percent H3PW12O40 modified aluminium-incorporated mesoporous KIT-6 In acetonitrile at 20℃; for 3h; Reagent/catalyst; Hosomi-Sakurai Reaction; Green chemistry; | 98% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
121-44-8
triethylamine

-
-
86759-27-5
tetraethylammonium fluorosulfonate

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride In acetonitrile at -20 - -14℃; under 13 - 500 Torr; Autoclave; | 97.5% |
| With fluorosulfonyl fluoride In chlorobenzene at 20℃; for 12h; Sealed tube; | 16% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
50653-07-1
trimethylsilyl triphenyl methyl ether

-
A
-
107-46-0
Hexamethyldisiloxane

-
B
-
968-39-8
Triphenylmethylethylether

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; | A n/a B 97% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
50653-07-1
trimethylsilyl triphenyl methyl ether

-
-
968-39-8
Triphenylmethylethylether

| Conditions | Yield |
|---|---|
| trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; | 97% |
| trimethylsilyl trifluoromethanesulfonate In dichloromethane at 0℃; for 0.5h; Product distribution; | 97% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
762-72-1
allyl-trimethyl-silane

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; 1-(n-butyl)-3-methylimidazolium triflate at 20℃; for 0.166667h; | 97% |
| Fe(OTs)3*6H2O In acetonitrile at 20℃; for 19h; | 84% |
| With bismuth(lll) trifluoromethanesulfonate In dichloromethane at 0℃; for 3h; | 82% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
768-92-3
1-adamantyl fluoride

-
-
6221-75-6
1-Adamantyl ethyl ether

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 24h; | 97% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
7727-18-6
vanadium(V) oxychloride

-
-
1801-77-0
oxovanadium(V) ethoxydichloride

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: (CH3)3SiCl; (N2); addn. of 2 equiv. of silane deriv. to CH2Cl2 soln. of vanadium compd. at room temp., stirring for 2 h at room temp.; evapn., elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride In acetonitrile at -19℃; under 14 - 400 Torr; Autoclave; | 97% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
791-31-1
triphenylhydroxysilane

-
-
799-53-1
1,1,1-trimethyl-3,3,3-triphenyl-disiloxane

| Conditions | Yield |
|---|---|
| 96.5% | |
| 96.5% | |
| 96.5% | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride In acetonitrile at -20 - -10℃; under 64 - 300 Torr; for 13.22h; Autoclave; | 96% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
4124-31-6
trichloroacetic acid anhydride

-
-
25436-07-1
trimethylsilyl trichloroacetate

| Conditions | Yield |
|---|---|
| With iron perchlorate hexahydrate at 25℃; for 0.5h; | 96% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
2051-49-2
n-hexanoic anhydride

-
-
14246-15-2
trimethylsilyl hexanoate

| Conditions | Yield |
|---|---|
| With CBV780 at 100℃; for 0.0833333h; Temperature; Microwave irradiation; | 96% |
-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
104-88-1
4-chlorobenzaldehyde

-
-
762-72-1
allyl-trimethyl-silane

-
-
1056626-90-4
4-ethoxy-4-(4-chlorophenyl)-1-butene

| Conditions | Yield |
|---|---|
| With cesium salt nanocrystal of tungstosilicic acid In acetonitrile at 20℃; for 3h; Sakurai reaction; | 95% |

-
-
1825-62-3
ethyl trimethylsilyl ether

-
-
122-00-9
para-methylacetophenone

-
-
762-72-1
allyl-trimethyl-silane

| Conditions | Yield |
|---|---|
| With 35 percent H3PW12O40 modified aluminium-incorporated mesoporous KIT-6 In acetonitrile at 20℃; for 2h; Reagent/catalyst; Hosomi-Sakurai Reaction; Green chemistry; | 95% |

Ethoxytrimethylsilane Consensus Reports
Reported in EPA TSCA Inventory.
Ethoxytrimethylsilane Specification
The IUPAC name of this chemical is ethoxy(trimethyl)silane. With the CAS registry number 1825-62-3 and EINECS 217-370-6, it is also named as Silane, ethoxytrimethyl-. The product's categories are Monoalkoxysilanes; Si (Classes of Silicon Compounds); Si-O Compounds. It is clear colorless liquid which reacts with water. When heated to decomposition it emits acrid smoke and irritating fumes. Additioanlly, this chemical should be sealed in the container and stored in the cool, well-ventilated and dry place. People should ensure that the workplace has well-ventilated equipment.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 2.15; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.15; (4)ACD/LogD (pH 7.4): 2.15; (5)ACD/BCF (pH 5.5): 25.37; (6)ACD/BCF (pH 7.4): 25.37; (7)ACD/KOC (pH 5.5): 352.2; (8)ACD/KOC (pH 7.4): 352.2; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 9.23 Å2; (13)Index of Refraction: 1.382; (14)Molar Refractivity: 35.67 cm3; (15)Molar Volume: 153.1 cm3; (16)Polarizability: 14.14×10-24 cm3; (17)Surface Tension: 16.7 dyne/cm; (18)Enthalpy of Vaporization: 30.35 kJ/mol; (19)Vapour Pressure: 115 mmHg at 25°C; (20)Rotatable Bond Count: 2; (21)Exact Mass: 118.081392; (22)MonoIsotopic Mass: 118.081392; (23)Topological Polar Surface Area: 9.2; (24)Heavy Atom Count: 7; (25)Complexity: 46.5.
Preparation of Ethoxytrimethylsilane: It can be obtained by chloro-trimethyl-silane and ethanol. This reaction needs reagent urea at temperature of 20 °C. The reaction time is 4 hours. The yield is 80.2%.
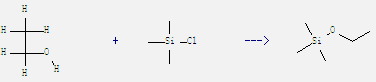
Uses of Ethoxytrimethylsilane: It is used in the synthesis of organic silicon compounds. And it is also used as water repellent. Besides, it can react with benzene-1,2,4,5-tetracarbonitrile to get 5-Methyl-1,2,4-benzenetricarbonitrile. This reaction needs solvent acetonitrile by irradiation. The reaction time is 2 hours. The yield is 85%.
.gif)
When you are using this chemical, please be cautious about it as the following:
It is not only harmful if swallowed, but also irritating to eyes, respiratory system and skin. And it is highly flammable, so people should keep away from sources of ignition. In case of contacting with eyes, rinse immediately with plenty of water and seek medical advice. After using it, take precautionary measures against static discharges. If you want to contact this product, you must wear suitable gloves and eye/face protection.
People can use the following data to convert to the molecule structure.
1. SMILES:O(CC)[Si](C)(C)C
2. InChI:InChI=1/C5H14OSi/c1-5-6-7(2,3)4/h5H2,1-4H3
3. InChIKey:RSIHJDGMBDPTIM-UHFFFAOYAV
The following are the toxicity data which has been tested.
Organism Test Type Route Reported Dose (Normalized Dose) Effect Source rat LCLo inhalation 4000ppm/8H (4000ppm) BEHAVIORAL: TREMOR
LUNGS, THORAX, OR RESPIRATION: DYSPNEA
GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDSJournal of Industrial Hygiene and Toxicology. Vol. 30, Pg. 332, 1948. rat LDLo oral 1400mg/kg (1400mg/kg) Journal of Industrial Hygiene and Toxicology. Vol. 30, Pg. 332, 1948.
Related Products
- Ethoxytrimethylsilane
- 1825-63-4
- 1825-64-5
- 182565-27-1
- 1825-68-9
- 1825-82-7
- 18259-15-9
- 18260-60-1
- 18260-66-7
- 18260-69-0
- 1826-11-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View