-
Name
Ethyl 2-oxo-4-phenylbutyrate
- EINECS 265-276-9
- CAS No. 64920-29-2
- Article Data33
- CAS DataBase
- Density 1.091 g/cm3
- Solubility
- Melting Point
- Formula C12H14O3
- Boiling Point 309 °C at 760 mmHg
- Molecular Weight 206.241
- Flash Point 140 °C
- Transport Information
- Appearance Pale yellow or yellow liquid
- Safety 23-24/25-36/37-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Oxo-4-phenylbutanoicacid ethyl ester;2-Oxo-4-phenylbutyric acid ethyl ester;4-Phenyl-2-oxobutyricacid ethyl ester;Ethyl 2-oxo-4-phenylbutanoate;Ethyl 3-benzylpyruvate;Ethyl 4-phenyl-2-ketobutyrate;Ethyl4-phenyl-2-oxobutanoate;Ethyl benzylpyruvate;
- PSA 43.37000
- LogP 1.75140
Synthetic route
-
-
93921-85-8
ethyl 2-hydroxy-4-phenylbutanoate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; calcium methylate In acetonitrile at 0 - 20℃; | 99% |
| With 4-hydroxy-TEMPO benzoate; sodium acetate In dichloromethane | 98% |
| With hydrogenchloride; sodium hypobromide In dichloromethane; water at 25℃; for 5h; | 94% |
-
-
55674-14-1
(Z)-ethyl 2-oxo-4-phenyl-3-butenoate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| 98% |

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium permanganate; sodium hydrogencarbonate; magnesium sulfate In water; acetone at 23℃; for 0.0833333h; | 93% |
-
-
943-73-7
D-homophenylalanine

-
-
141-78-6
ethyl acetate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hypochlorite; tetrabutylammomium bromide In water at 0 - 40℃; Product distribution / selectivity; | 84.1% |
-
-
17451-20-6, 55674-14-1, 55629-96-4
(E)-ethyl-2-oxo-4-phenylbut-3-enoate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 1-propyl-1,4-dihydronicotinamide; magnesium(II) perchlorate In acetonitrile Ambient temperature; | 82% |
-
-
64-17-5
ethanol

-
-
67333-73-7
1-nitro-4-phenylbutan-2-one

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 1-[4-(diacetoxyiodo)benzyl]-3-methylimidazolium tetrafluoroborate for 1h; Reflux; | 80% |
-
-
103-63-9
1-phenyl-2-bromoethane

-
-
95-92-1
oxalic acid diethyl ester

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-phenyl-2-bromoethane With magnesium In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: oxalic acid diethyl ester In tetrahydrofuran at -78℃; for 2h; Inert atmosphere; | 79% |
| Stage #1: 1-phenyl-2-bromoethane With magnesium In tetrahydrofuran for 1h; Reflux; Inert atmosphere; Stage #2: oxalic acid diethyl ester In tetrahydrofuran at -10℃; for 1.5h; Inert atmosphere; Stage #3: With hydrogenchloride In tetrahydrofuran; water Inert atmosphere; | 60% |
| Stage #1: 1-phenyl-2-bromoethane With iodine; magnesium In tetrahydrofuran for 1h; Reflux; Stage #2: oxalic acid diethyl ester In tetrahydrofuran at -78 - 0℃; for 1h; | 50% |
-
-
3277-89-2
phenethylmagnesium bromide

-
-
220332-87-6
ethyl 3,5-dimethyl-1-pyrazolylglyoxylate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In diethyl ether at -90℃; for 1h; | 76% |
-
-
64-17-5
ethanol

-
-
70102-85-1
3-oxo-5-phenyl-pentanenitrile

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene In dichloromethane for 2h; Reflux; | 57% |
-
-
3277-89-2
phenethylmagnesium bromide

-
-
95-92-1
oxalic acid diethyl ester

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -10℃; for 1h; | 55% |
| In diethyl ether at -10℃; for 2h; |
-
-
70187-08-5
2,2-Bis-ethylsulfanyl-4-phenyl-butyric acid ethyl ester

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With water; calcium carbonate; mercury dichloride |
-
-
64-17-5
ethanol

-
-
645-59-0
dihydrocinnamonitrile

-
-
33577-16-1
methyl methylsulfinylmethyl sulfide

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| (i) NaH, (ii) /BRN= 1718733/, CuCl2, CuO; Multistep reaction; |

-
-
710-11-2
2-oxo-4-phenylbutyric acid

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In ethanol; sulfuric acid for 5h; Heating; Yield given; |
-
-
46460-23-5, 46460-24-6, 82830-84-0, 124044-66-2
ethyl L-2-amino-4-phenylbutyrate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; 3-chloro-benzenecarboperoxoic acid; isopentyl nitrite 1.) chloroform, 2.) 30 min; Yield given. Multistep reaction; | |
| With sodium hypochlorite Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride 1.) ether, room temperature, 24 h, 2.) ether, 0 deg C, 3 h; Yield given. Multistep reaction; |
-
-
90878-19-6
phenethylmagnesium chloride

-
-
95-92-1
oxalic acid diethyl ester

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In diethyl ether Grignard reaction; |
-
-
617-35-6
2-oxo-propionic acid ethyl ester

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 35℃; for 144h; |
-
-
4263-93-8
2-hydroxy-4-phenylbutanoic acid

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93 percent / SOCl2 / 10 h / Ambient temperature 2: 87 percent Chromat. / CrO3-pyridine / CH2Cl2 / 2.5 h / other reagents: K3Cl>, CrO3/Al2O3 View Scheme |
-
-
100-52-7
benzaldehyde

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 78 percent Chromat. / NaOH / temp. <=10 deg C 2: 77 percent / hydrogen / Ni-Raney / ethanol; H2O / 4 h / Ambient temperature; other catalyst: Ni on Kieselguhr 3: 93 percent / SOCl2 / 10 h / Ambient temperature 4: 87 percent Chromat. / CrO3-pyridine / CH2Cl2 / 2.5 h / other reagents: K3Cl>, CrO3/Al2O3 View Scheme |

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 77 percent / hydrogen / Ni-Raney / ethanol; H2O / 4 h / Ambient temperature; other catalyst: Ni on Kieselguhr 2: 93 percent / SOCl2 / 10 h / Ambient temperature 3: 87 percent Chromat. / CrO3-pyridine / CH2Cl2 / 2.5 h / other reagents: K3Cl>, CrO3/Al2O3 View Scheme |
-
-
2021-28-5
ethyl dihydrocinnamate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) NaOMe; 2) 15 percent H2SO4 / 1) MeOH, 60-70 deg C, 1.5 h; 2) reflux 15 h 2: ethanol; H2SO4 / 5 h / Heating View Scheme |
-
-
70-23-5
ethyl Bromopyruvate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 85 percent / HCO3(1-) 2: 41 percent / xylene 3: 82 percent / 1-n-propyl-1,4-dihydronicotinamide (PNAH, 4) / Mg(ClO4)2 / acetonitrile / Ambient temperature View Scheme |
-
-
13321-61-4
ethyl 2-oxo-3-(triphenylphosphoranylidene)propanoate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 41 percent / xylene 2: 82 percent / 1-n-propyl-1,4-dihydronicotinamide (PNAH, 4) / Mg(ClO4)2 / acetonitrile / Ambient temperature View Scheme |

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) THF, (ii) /BRN= 385653/ 2: H2O, CaCO3, HgCl2 View Scheme |
-
-
64-17-5
ethanol

-
-
710-11-2
2-oxo-4-phenylbutyric acid

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine; methanesulfonyl chloride In tetrahydrofuran at 0 - 20℃; |
-
-
95-92-1
oxalic acid diethyl ester

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at -78℃; |
-
-
122-78-1
phenylacetaldehyde

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 0.5 h / -78 °C / Inert atmosphere 1.2: 5.75 h / -78 - 20 °C / Inert atmosphere 2.1: acetic acid; cesium fluoride / acetonitrile / 5 h / 0 - 20 °C / Inert atmosphere View Scheme |

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With acetic acid; cesium fluoride In acetonitrile at 0 - 20℃; for 5h; Inert atmosphere; |
-
-
883581-67-7
diazo(trimethylsilyl)methyl magnesium bromide

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In various solvents at -78℃; for 1.5h; | 100% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
292858-05-0
ethyl 3-bromo-2-oxo-4-phenylbutanoate

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In chloroform; ethyl acetate for 6h; Reflux; | 99% |
| With bromine In tetrachloromethane; chloroform | 97% |
| With bromine In tetrachloromethane; chloroform at 20℃; for 1h; | 95% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
60144-26-5
formaldehyde N,N-tetramethylenehydrazone

-
-
1330591-15-5
(E)-ethyl 2-hydroxy-4-phenyl-2-[(pyrrolidin-1-ylimino)methyl]butanoate

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxo-4-phenylbutanoic acid ethyl ester With 1,3-bis(3,5-bis(trifluoro-ethyl)phenyl)thiourea In water at 20℃; for 0.25h; Stage #2: formaldehyde N,N-tetramethylenehydrazone In water | 99% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Catalytic behavior; Solvent; Reagent/catalyst; Inert atmosphere; Sealed tube; | 99% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
52719-93-4
(I)-menthyloxycarbonylhydroxamic acid

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; Sealed tube; | 95% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With hydrogen; Cinchonin In acetic acid at 25℃; under 7500.75 Torr; for 12h; enantioselective reaction; | 94.4% |
-
-
51-80-9
bis-(dimethylamino)methane

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With acetic anhydride In ethyl acetate; N,N-dimethyl-formamide | 93% |
-
-
3669-32-7
cinnamic acid N-hydroxylamide

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; Sealed tube; | 93% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
90315-82-5
ethyl (R)-2-hydroxy-4-phenylbutyrate

| Conditions | Yield |
|---|---|
| With D-glucose at 30℃; for 12h; pH=7.0; aq. phosphate buffer; optical yield given as %ee; enantioselective reaction; | 92% |
| With D-glucose In phosphate buffer at 30℃; for 26h; pH=7; | 91% |
| With glucose dehydrogenase; D-glucose; recombinant reductase CgKR2 from Candida glabrata; NADP; sodium carbonate at 30℃; for 5h; pH=6; Kinetics; Reagent/catalyst; Solvent; Temperature; Time; aq. phosphate buffer; Enzymatic reaction; optical yield given as %ee; enantioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; Sealed tube; | 92% |
-
-
61550-02-5
(furan-2-yloxy)-trimethylsilane

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
1140920-60-0
(R,R)-ethyl 2-hydroxy-2-(5-oxo-2,5-dihydrofuran-2-yl)-4-phenylbutanoate

| Conditions | Yield |
|---|---|
| With 3,3,3-Trifluoropropanol; copper(II) bis(trifluoromethanesulfonate); (S)-N-[2-(2,4,6-triisopropylbenzylamino)phenyl]-S-methyl-S-phenylsulfoximine In diethyl ether at 20℃; Mukaiyama aldol reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 91% |
| With 2,2,2-trifluoroethanol; copper(II) bis(trifluoromethanesulfonate); (S)-N-[2-(2,4,6-triisopropylbenzylamino)phenyl]-S-methyl-S-phenylsulfoximine In diethyl ether at 20℃; Vinylogous Mukaiyama-type aldol reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 91% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
124888-60-4, 124988-64-3, 143615-31-0, 1199-97-9
4-phenylbutane-1,2-diol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 0.166667h; | 91% |
| Stage #1: 2-oxo-4-phenylbutanoic acid ethyl ester With sodium tetrahydroborate In methanol Inert atmosphere; Stage #2: With hydrogenchloride In water Inert atmosphere; | 72% |
-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
132489-33-9
(S)-2-benzyloxy-3-pentanone

-
-
1520094-30-7
(3R,4S,5S)-ethyl 5-benzyloxy-2-hydroxy-3-methyl-2(2-phenylethyl)-4-oxohexanoate

| Conditions | Yield |
|---|---|
| Stage #1: (S)-2-benzyloxy-3-pentanone With titanium tetrachloride; N-ethyl-N,N-diisopropylamine In dichloromethane at -78℃; for 0.666667h; Inert atmosphere; Stage #2: 2-oxo-4-phenylbutanoic acid ethyl ester In dichloromethane at -20℃; for 3h; Inert atmosphere; diastereoselective reaction; | 91% |
-
-
36016-38-3
tert-Butyl N-hydroxycarbamate

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; Sealed tube; | 91% |
-
-
75-52-5
nitromethane

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With rac-1',2',3',4'-tetrahydro-1,1'-bisisoquinoline In tetrahydrofuran at 20℃; for 24h; Henry Nitro Aldol Condensation; | 90% |
| With copper(II) bis(trifluoromethanesulfonate); 2,2'-isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline]; triethylamine at 50℃; |
-
-
54-85-3
isoniazid

-
-
64920-29-2
2-oxo-4-phenylbutanoic acid ethyl ester

-
-
433719-25-6
ethyl 2-oxo-4-phenylbutyrate isonicotinoylhydrazone

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: dibenzyl azodicarboxylate; 2-oxo-4-phenylbutanoic acid ethyl ester With 1-(3,5-bis(trifluoromethyl)phenyl)-3-((1R,2R)-2-(piperidin-1-yl)cyclohexyl)thiourea In 1,3,5-trimethyl-benzene at -5℃; for 11h; Molecular sieve; Stage #2: With L-Selectride In 1,3,5-trimethyl-benzene at -30℃; for 2h; Molecular sieve; enantioselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; 3-hydroxyquinuclidine In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; Sealed tube; | 90% |
Ethyl 2-oxo-4-phenylbutyrate Chemical Properties
Following is the structure of Ethyl 2-oxo-phenylbutrate (CAS NO.64920-29-2):
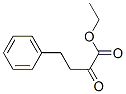
IUPAC Name: Ethyl 2-oxo-4-phenylbutanoate
Molecular Formula: C12H14O3
Molecular Weight: 206.237760 g/mol
Appearance: yellow liquid
BRN: 2725083
EINECS: 265-276-9
Index of Refraction: 1.506
Molar Refractivity: 56.13 cm3
Molar Volume: 188.8 cm3
Density: 1.091 g/cm3
Flash Point: 140 °C
Surface Tension: 39.2 dyne/cm
Enthalpy of Vaporization: 54.97 kJ/mol
Boiling Point: 309 °C at 760 mmHg
Vapour Pressure: 0.000657 mmHg at 25 °C
Product Categories: Intermediates of Sertraline; Pharmaceutical Intermediates; Aromatics; (intermediate of lisinopril ); C12 to C63; Carbonyl Compounds; Esters
SMILES: O=C(OCC)C(=O)CCc1ccccc1
InChI: InChI=1/C12H14O3/c1-2-15-12(14)11(13)9-8-10-6-4-3-5-7-10/h3-7H,2,8-9H2,1H3
InChIKey: STPXIOGYOLJXMZ-UHFFFAOYAY
Ethyl 2-oxo-4-phenylbutyrate Uses
Ethyl 2-oxo-phenylbutrate with cas registry number of 64920-29-2 is used as an intermediate in organic synthesis and pharmaceutical.
Ethyl 2-oxo-4-phenylbutyrate Safety Profile
Safety Information of Ethyl 2-oxo-phenylbutrate (CAS NO. 64920-29-2):
Hazard Codes:Xi 
Risk Statements:36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:23-24/25-36/37-26
23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24/25:Avoid contact with skin and eyes
36/37:Wear suitable protective clothing and gloves
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany:3
Ethyl 2-oxo-4-phenylbutyrate Specification
Ethyl 2-oxo-phenylbutrate , its cas register number 64920-29-2. It also can be called Benzenebutanoic acid, .alpha.-oxo-, ethyl ester ; and Benzenebutanoic acid, alpha-oxo-, ethyl ester .
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- Ethyl (2R)-2-(4-hydroxyphenoxy)propanoate
- Ethyl (2R)-2,3-epoxypropanoate
- 64920-31-6
- 6492-48-4
- 64928-87-6
- 64928-88-7
- 6493-05-6
- 64930-85-4
- 64938-84-7
- 6493-92-1
- 6494-19-5
- 64942-17-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View