-
Name
ETHYL THIOGLYCOLATE
- EINECS 210-800-3
- CAS No. 623-51-8
- Article Data30
- CAS DataBase
- Density 1.072 g/cm3
- Solubility Not miscible or difficult to mix in water.
- Melting Point -80 °C
- Formula C4H8O2S
- Boiling Point 157.8 °C at 760 mmHg
- Molecular Weight 120.172
- Flash Point 47.8 °C
- Transport Information UN 1992 3/PG 3
- Appearance colourless liquid
- Safety 26-36/37/39-45
- Risk Codes 10-25-36/38
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Ethyl mercaptoacetate;Ethyl thioglycolate;Ethyl a-mercaptoacetate;Ethylthiomethyl acetate;Mercaptoacetic acid ethylester;NSC 8834;Sulfanylacetic acid ethyl ester;Thioglycolic acid ethyl ester;Ethyl 2-mercaptoacetate;Ethoxycarbonylmethanethiol;2-Mercaptoacetic acid ethyl ester;Aceticacid, mercapto-, ethyl ester (7CI,8CI,9CI);
- PSA 65.10000
- LogP 0.47930
Synthetic route

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In chloroform esterified azeotropically; | 72% |
| With LEWATIT K2621 In toluene for 72h; Heating; | 58% |
| With magnesium sulfate; toluene-4-sulfonic acid for 16h; Inert atmosphere; Reflux; | 36% |
-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
27032-03-7
1-(ethoxycarbonylmethyl)pyridinium chloride

-
A
-
17281-70-8
pyridinium cyanoi(ethoxycarbonyl)methylide

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 63% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
42508-60-1
1-(2-oxopropyl)pyridinium chloride

-
A
-
37026-10-1
Cyano-acetyl-pyridinium-methylid

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 58% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
129170-15-6
1-ethoxycarbonylmethyl-4-methylpyridinium chloride

-
A
-
84802-40-4
C11H12N2O2

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 53% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
129170-16-7
1-Ethoxycarbonylmethyl-3,5-dimethyl-pyridinium; chloride

-
A
-
84802-41-5
C12H14N2O2

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 51% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
20517-71-9
1-(2-oxo-2-phenylethyl)pyridin-1-ium chloride

-
A
-
50737-38-7, 17281-69-5
1-(1-cyano-2-oxo-2-phenyl-ethyl)-pyridinium betaine

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 45% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
129170-17-8
1-acetonyl-3,5-dimethylpyridinium chloride

-
A
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A n/a B 42% |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
115260-53-2
1-acetonyl-4-methylpyridinium chloride

-
A
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A n/a B 40% |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
105757-72-0
1-[2-oxo-2-(phenyl)ethyl]-4-methylpyridinium chloride

-
A
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A n/a B 37% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A n/a B 28% |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
120616-12-8
1-(cyanomethyl)-3,5-dimethylpyridinium chloride

-
A
-
26960-15-6
3,5-dimethylpyridinium dicyanomethylide

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 18% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
A
-
3189-57-9
4-methylpyridinium dicyanomethylide

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 15% B n/a |

-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
17281-59-3
2-pyridin-1-ium-1-ylacetonitrile chloride

-
A
-
27032-01-5
pyridinium 1-dicyanomethylide

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform Ambient temperature; | A 7% B n/a |


| Conditions | Yield |
|---|---|
| With phosphoric acid beim Destillieren; |

| Conditions | Yield |
|---|---|
| With ethanol; 6-methyl-2,4-dinitrophenol durch Oxydations-Reduktionsfermente im Sonnenlicht bei pH 7.2; |

| Conditions | Yield |
|---|---|
| zerfaellt in der Waerme; |
-
-
61713-25-5
4,4-diethyl-3,5-dithia-heptanedioic acid diethyl ester

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| Destillation im Vakuum; | |
| Destillation im Vakuum; |
-
-
860540-06-3
4,4-dipropyl-3,5-dithia-heptanedioic acid diethyl ester

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| Destillation im Vakuum; |
-
-
860541-76-0
4,4-dibutyl-3,5-dithia-heptanedioic acid diethyl ester

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| Destillation im Vakuum; |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
105-36-2
ethyl bromoacetate

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With pyridine; phosphorous (V) sulfide anschliessend Behandeln mit Wasser; |

| Conditions | Yield |
|---|---|
| Veresterung; |

| Conditions | Yield |
|---|---|
| With ethanol; potassium hydrosulfide | |
| With hydrosulfide exchange resin (from Amberlite IRA-400); triethylamine hydrochloride In methanol for 1h; Ambient temperature; | 97 % Chromat. |

| Conditions | Yield |
|---|---|
| With ethanol |
-
-
84257-71-6
1-(ethoxycarbonylmethylthio)-4-methylphthalazine

-
A
-
5004-48-8
1-methyl-3,4-dihydro-4-oxophthalazine

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water at 25℃; Rate constant; var. pH; |
-
-
82408-32-0
(1-Hydroxy-2-oxo-2-phenyl-ethylsulfanyl)-acetic acid ethyl ester

-
A
-
1075-06-5
2,2-dihydroxy-1-phenyl-ethanone

-
B
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In water; acetonitrile at 30℃; Equilibrium constant; |
-
-
5349-28-0
thiocyanato-acetic acid ethyl ester

-
-
86119-84-8, 7664-38-2
phosphoric acid

-
-
7732-18-5
water

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
78594-34-0
2-(acetylthio)acetate ethyl ester

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With water |

-
A
-
1665-65-2
diethyl 2,2'-disulfanediyldiacetate

-
B
-
623-51-8
ethyl 2-sulfanylacetate

-
C
-
741268-81-5, 741268-84-8, 133227-82-4, 133320-10-2, 133320-11-3, 133869-47-3, 134054-62-9, 134932-61-9, 149820-05-3, 149820-06-4, 157903-26-9, 175779-21-2, 115383-22-7, 958017-68-0, 958017-71-5, 958017-74-8, 958017-77-1, 958017-80-6, 140630-87-1, 140694-21-9, 151716-50-6, 157008-89-4, 220062-79-3, 220062-83-9
[5,6]fullerene-C70

| Conditions | Yield |
|---|---|
| at 50℃; Temperature; |

-
-
6602-54-6
2-chloro-3-pyridinecarbonitrile

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
52505-46-1
ethyl 3-aminothieno[2,3-b]pyridine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-sulfanylacetate With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; for 0.25h; Stage #2: 2-chloro-3-pyridinecarbonitrile In dimethyl sulfoxide; mineral oil at 20℃; for 3h; | 100% |
| With sodium carbonate In ethanol at 90℃; for 2.5h; | 94% |
| With sodium carbonate In ethanol for 4.5h; Heating / reflux; | 93.2% |
-
-
115975-33-2
N,N-Dimethyl-2,4-bis(trifluoroacetyl)-1-naphthylamine

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
140666-92-8
2-ethoxycarbonyl-5-trifluoroacetyl-3-trifluoromethylnaphtho<1,2-b>thiophene

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Heating; | 100% |
-
-
930-96-1
3-bromo-2-thiophenecarboxaldehyde

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
201004-08-2
ethyl thieno[3,2,-b]thiophene-2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60 - 70℃; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 25 - 30℃; Inert atmosphere; Large scale; | 97.9% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 72h; | 91% |
-
-
117-80-6
2,3-Dichloro-1,4-naphthoquinone

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
916250-47-0
ethyl 2-(3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-ylthio)acetate

| Conditions | Yield |
|---|---|
| With water at 50℃; for 2h; | 100% |
| In water at 50℃; for 2h; | 100% |
| In ethanol at 80 - 90℃; | 97% |
-
-
1009334-65-9
4-chloro-3-cyano-5-fluoropyridine

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
1008523-97-4
3-amino-7-fluoro-thieno[3,2-c]pyridine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 40℃; for 1h; | 100% |
-
-
17417-09-3
2-fluoro-5-nitrobenzonitrile

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
27697-60-5
ethyl (3-amino-5-nitrobenzothiophen)-2-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In dimethyl sulfoxide | 100% |
| Stage #1: ethyl 2-sulfanylacetate With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; for 0.333333h; Stage #2: 2-fluoro-5-nitrobenzonitrile In dimethyl sulfoxide; mineral oil at 20℃; for 3h; | 93% |
| With triethylamine In dimethyl sulfoxide at 20℃; for 3h; | 90% |
-
-
3518-87-4
1-benzoyl-4-oxo-piperidine-3-carboxylic acid methyl ester

-
-
623-51-8
ethyl 2-sulfanylacetate

-
A
-
64281-03-4
1-benzoyl-4-ethoxycarbonylmethylsulfanyl-1,2,5,6-tetrahydro-pyridine-3-carboxylic acid methyl ester

-
B
-
64281-02-3
5-benzoyl-3-hydroxy-4,5,6,7-tetrahydro-thieno[3,2-c]pyridine-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In ethanol | A n/a B 100% |

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
178876-82-9
6-amino-5-bromo-pyridine-2-carboxylic acid methyl ester

-
-
443956-13-6
methyl 3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]thiazine-6-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-sulfanylacetate With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Stage #2: 6-amino-5-bromo-pyridine-2-carboxylic acid methyl ester In N,N-dimethyl-formamide at 20℃; | 100% |
| Stage #1: ethyl 2-sulfanylacetate With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Stage #2: 6-amino-5-bromo-pyridine-2-carboxylic acid methyl ester In N,N-dimethyl-formamide at 20℃; for 16h; | |
| Stage #1: ethyl 2-sulfanylacetate With sodium hydride In DMF (N,N-dimethyl-formamide) at 0℃; for 1h; Stage #2: 6-amino-5-bromo-pyridine-2-carboxylic acid methyl ester In DMF (N,N-dimethyl-formamide) at 20℃; for 16h; |
-
-
18269-47-1
allyldimethylethoxysilane

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
1024594-59-9
[3-(ethoxydimethylsilanyl)propylsulfanyl]acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 100℃; for 16h; Inert atmosphere; | 100% |
-
-
1351238-40-8
3-bromo-6-(furan-2-yl)pyrazine-2-carbonitrile

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
1351238-41-9
ethyl 7-amino-2-(furan-2-yl)thieno[2,3-b]pyrazine-6-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol at 20 - 50℃; for 3h; | 100% |
| With sodium carbonate In ethanol at 20 - 50℃; for 3h; | 10% |
-
-
10135-00-9
3-bromobenzo[b]thiophene-2-carbaldehyde

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
35616-45-6
ethyl thieno[3,2-b][1]benzothiophene 2-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 50h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 72h; | 92% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With potassium carbonate In N,N-dimethyl-formamide |
-
-
453-71-4
3-nitro-4-fluorobenzoic acid

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
204863-51-4
4-ethoxycarbonylmethylsulfanyl-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium acetate In water at 90℃; for 24h; Inert atmosphere; | 100% |
-
-
1397833-51-0
(2-amino-3-methylphenyl) (benzotriazole-1-yl)methanone

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
1476066-75-7
ethyl 2-(2-amino-3-methylbenzoylthio)acetate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; | 100% |

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethylguanidine In N,N-dimethyl-formamide at 23℃; for 16h; | 100% |
-
-
1542437-92-2
3-bromo-5-(4-methoxyphenyl)thiophene-2-carbaldehyde

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 50h; | 100% |
-
-
1309598-16-0
3-bromo-5-(4-(trifluoromethyl)phenyl)thiophene-2-carbaldehyde

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 50h; | 100% |
-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 0 - 20℃; | 100% |
| With potassium tert-butylate In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; | 100% |
-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
159730-72-0
2-bromo-4-methyl-5-nitrobenzaldehyde

-
-
159730-73-1
ethyl 6-methyl-5-nitrobenzo[b]thiophene-2-carboxylate

| Conditions | Yield |
|---|---|
| With sodium In ethanol for 3h; Inert atmosphere; Reflux; | 99.8% |
| With sodium ethanolate 1.) EtOH, 5 deg C, 20 min, 2.) EtOH, reflux, 3 h; Yield given. Multistep reaction; |
-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
10191-60-3
dimethyl N-cyanodithioiminocarbonate

-
-
39736-29-3
ethyl 4-amino-2-(methylthio)thiazol-5-carboxylate

| Conditions | Yield |
|---|---|
| With diisopropylamine In N,N-dimethyl-formamide at 100℃; for 5h; | 99% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 100℃; | 89% |
| With triethylamine In N,N-dimethyl-formamide | |
| With triethylamine In N,N-dimethyl-formamide at 100℃; for 2h; |
-
-
286-20-4
cyclohexane-1,2-epoxide

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
82622-17-1
trans-2-hydroxycyclohexyl(carboethoxy)methyl sulfide

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 25h; Heating; | 99% |
-
-
110657-96-0
2-((R)-oxiran-2-ylmethoxy)tetrahydro-2H-pyran

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
106851-44-9
[(R)-2-Hydroxy-3-(tetrahydro-pyran-2-yloxy)-propylsulfanyl]-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| sodium ethanolate In ethanol at 22℃; | 99% |
-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
70-11-1
α-bromoacetophenone

-
-
66560-19-8
ethyl 2-((2-oxo-2-phenylethyl)thio)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 20℃; Inert atmosphere; | 99% |
| With sodium hydroxide In methanol for 4h; | 60% |
| With sodium carbonate In ethanol at 20℃; |
-
-
5147-80-8
[Bis(methylthio)methylene]malononitrile

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
116170-90-2
3-amino-4-cyano-5-(methylthio)thiophene-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In methanol for 2h; Reflux; | 99% |
| With triethylamine In methanol for 2h; Reflux; | 99% |
| With triethylamine In ethanol at 0 - 20℃; for 12h; | 72.6% |
-
-
211678-96-5
3-chloroisonicotinic acid ethyl ester

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
1008523-91-8
ethyl 3-hydroxythieno [2,3-c]pyridine-2-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 5 - 20℃; for 18.67h; | 99% |
| In N,N-dimethyl-formamide; mineral oil at 20℃; for 3.25h; Cooling with ice; | 70% |
-
-
133116-83-3
2-fluoro-6-(trifluoromethyl)benzonitrile

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 0 - 20℃; | 99% |
| With potassium tert-butylate In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; | 99% |
-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-sulfanylacetate With sodium hydride In tetrahydrofuran at 0 - 20℃; for 0.333333h; Inert atmosphere; Stage #2: N-(benzyloxy)formimidoyl chloride In tetrahydrofuran at 20℃; for 5h; Inert atmosphere; | 99% |

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With Trimethylsilanol; (S)-N-(2-pyridinesulfonyl)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)methanamine In toluene at -80℃; for 8h; Inert atmosphere; enantioselective reaction; | 99% |

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With Trimethylsilanol; (S)-N-(2-pyridinesulfonyl)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)methanamine In toluene at -80℃; for 8h; Inert atmosphere; enantioselective reaction; | 99% |

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With Trimethylsilanol; (S)-N-(2-pyridinesulfonyl)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)methanamine In toluene at -80℃; for 8h; Inert atmosphere; enantioselective reaction; | 99% |

-
-
623-51-8
ethyl 2-sulfanylacetate

| Conditions | Yield |
|---|---|
| With Trimethylsilanol; (S)-N-(2-pyridinesulfonyl)-(6-methoxyquinolin-4-yl)(8-vinylquinuclidin-2-yl)methanamine In toluene at -80℃; for 8h; Inert atmosphere; enantioselective reaction; | 99% |
Ethyl mercaptoacetate Specification
The Acetic acid,2-mercapto-, ethyl ester, with the CAS registry number 623-51-8, is also known as Thioglycolic acid ethyl ester. Its EINECS number is 210-800-3. This chemical's molecular formula is C4H8O2S and molecular weight is 120.17. What's more, its systematic name is ethyl sulfanylacetate. It is air sensitive. It should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides, static electricity and fire. It is used as a reagent to determinate iron content.
Physical properties of Acetic acid,2-mercapto-, ethyl ester are: (1)ACD/LogP: 1.18; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.18; (4)ACD/LogD (pH 7.4): 1.14; (5)ACD/BCF (pH 5.5): 4.66; (6)ACD/BCF (pH 7.4): 4.21; (7)ACD/KOC (pH 5.5): 104.72; (8)ACD/KOC (pH 7.4): 94.67; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 51.6 Å2; (13)Index of Refraction: 1.452; (14)Molar Refractivity: 30.25 cm3; (15)Molar Volume: 112 cm3; (16)Polarizability: 11.99×10-24cm3; (17)Surface Tension: 32.6 dyne/cm; (18)Density: 1.072 g/cm3; (19)Flash Point: 47.8 °C; (20)Enthalpy of Vaporization: 39.45 kJ/mol; (21)Boiling Point: 157.8 °C at 760 mmHg; (22)Vapour Pressure: 2.7 mmHg at 25°C.
Preparation: this chemical can be prepared by thioglycollicacid and ethanol by heating. This reaction will need solvent sulfuric acid with the reflux time of 24 hours. The product is gained by collecting the cut fraction of the temperature of 155-158 °C at atmospheric fractionation.
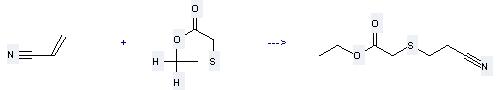
Uses of Acetic acid,2-mercapto-, ethyl ester: it can be used to produce (2-cyano-ethylsulfanyl)-acetic acid ethyl ester at the ambient temperature. It will need reagent sodium ethoxide with the reaction time of 14 hours. The yield is about 90%.
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable, so you should keep it away from sources of ignition - No smoking. It is toxic if swallowed. It is irritating to eyes and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1S/C4H8O2S/c1-2-6-4(5)3-7/h7H,2-3H2,1H3
(2)InChIKey: PVBRSNZAOAJRKO-UHFFFAOYSA-N
(3)Canonical SMILES: CCOC(=O)CS
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 100mg/kg (100mg/kg) | National Technical Information Service. Vol. AD277-689. | |
| rat | LD50 | intraperitoneal | 176mg/kg (176mg/kg) | Zeitschrift fuer die Gesamte Hygiene und Ihre Grenzgebiete. Vol. 20, Pg. 575, 1974. | |
| rat | LD50 | oral | 178mg/kg (178mg/kg) | Zeitschrift fuer die Gesamte Hygiene und Ihre Grenzgebiete. Vol. 20, Pg. 575, 1974. |
Related Products
- Ethyl (chloroformyl)acetate
- Ethyl (chlorosulfonyl)acetate
- Ethyl (E)-7-[4-(4'-fluorophenyl)-2-(cyclopropyl)-3-quinolinyl]-5-hydroxy-3-oxo-6-heptenoate
- Ethyl (E)-crotonate
- Ethyl (E)-hex-2-enoate
- Ethyl [1,2,4]triazolo[1,5-a]pyridine-2-carboxylate
- Ethyl 1-((pyridin-4-yl)methyl)piperidine-4-carboxylate
- Ethyl 1-(2,4-difluorophenyl)-7-chloro-6-fluoro-4-oxopyridino[2,3-b]pyridine-3-carboxylate
- Ethyl 1-(2,5-dichlorophenyl)-1H-imidazole-5-carboxylate
- Ethyl 1-(2-chloroacetyl)piperidine-4-carboxylate
- 623-53-0
- 62353-75-7
- 62356-27-8
- 623-56-3
- 62356-37-0
- 623563-74-6
- 623564-38-5
- 623564-49-8
- 623565-57-1
- 623-57-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View