-
Name
Ethyl propionate
- EINECS 203-291-4
- CAS No. 105-37-3
- Article Data249
- CAS DataBase
- Density 0.891 g/cm3
- Solubility 25 g/L (15 °C)
- Melting Point -73 °C(lit.)
- Formula C5H10O2
- Boiling Point 95.9 °C at 760 mmHg
- Molecular Weight 102.133
- Flash Point 12.2 °C
- Transport Information UN 1195 3/PG 2
- Appearance Colourless liquid with a fruity, rum-like, ethereal odour
- Safety 16-23-24-29-33
- Risk Codes 11
-
Molecular Structure
-
Hazard Symbols
 F
F
- Synonyms Propionate dethyle [French];Propionic ester;Propionic acid, ethyl ester;Propionic ether;Ethyl propionate (natural);Ethylester kyseliny propionove [Czech];Propionate dethyle;FEMA No. 2456;Ethyl propanoate;Ethyl Propionat;Ethyl Propionate , Natural;Natural Ethyl Propionate;Ethyl Propionate Natural;Ethylpropionate;Ethyl propionate/Nat.;
- PSA 26.30000
- LogP 0.95950
Synthetic route
-
-
13837-45-1
1-ethoxy-1-cyclopropanol

-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With lithium cyanide In tetrahydrofuran for 2h; Product distribution; Heating; | 100% |
| at 100℃; |

| Conditions | Yield |
|---|---|
| With Dowex 50W×2 hydrogen form resin at 107 - 110℃; Reagent/catalyst; Autoclave; Large scale; | 99.13% |
| With iron(III) sulfate; sulfuric acid for 2h; Heating; | 96% |
| With polymer supported sulfonated magnetic resin In toluene at 20 - 70℃; for 0.75h; | 88% |

| Conditions | Yield |
|---|---|
| at 120℃; for 24h; | 98% |
-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With water; lithium diisopropyl amide In tetrahydrofuran 1) -78 deg C, 30 min 2) 30 min; | 98% |
| With DMBI In diethyl ether for 2h; Heating; | 90% |
| With tert-Butyl peroxybenzoate; tri-n-butylphosphine-borane complex In chlorobenzene at 110℃; for 1h; | 90% |

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane for 0.0666667h; | 95% |

| Conditions | Yield |
|---|---|
| With 1H-imidazole In neat (no solvent) at 115℃; for 0.0666667h; Temperature; Wavelength; Microwave irradiation; | 91% |

| Conditions | Yield |
|---|---|
| With [MgBr](1+)*[n-Bu2SnBrIH](1-) In tetrahydrofuran at 20℃; for 0.5h; | 90% |
| With 1,3-dimethyl-2-imidazolidinone; Dimethylphenylsilane In [D3]acetonitrile at 80℃; for 10h; | 85% |
| With hydrogen In ethanol at 25℃; under 15001.5 Torr; for 12h; regioselective reaction; | 81% |

| Conditions | Yield |
|---|---|
| With 3-(7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)methyl-1-(2,4,6-trimethylphenyl)-1H-imidazol-3-ium iodine salt; caesium carbonate In toluene at 60℃; for 3h; | 84% |
| With C22H29N2O(1+)*I(1-); caesium carbonate In toluene at 60℃; for 3h; | 84% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform at 20℃; for 14h; Baeyer-Villiger oxidation; | 82% |
| With dihydrogen peroxide; acetic acid In toluene at 70℃; for 6h; | 36.8% |
| With Candida antarctica lipase; dihydrogen peroxide; n-tetradecanoic acid In toluene for 144h; | 20 % Chromat. |

| Conditions | Yield |
|---|---|
| With N-benzyl-trimethylammonium hydroxide In dimethyl sulfoxide at 80℃; for 4h; Decarboxylation; | 79% |
| With zinc(II) chloride | |
| With platinum at 200℃; Hydrogenation; | |
| Multi-step reaction with 2 steps 1: potassium hydroxide / ethanol / 72 h / 20 °C 2: 1H-imidazole / neat (no solvent) / 0.07 h / 115 °C / Microwave irradiation View Scheme |

| Conditions | Yield |
|---|---|
| With 1,2-diamino-benzene for 4h; Heating; | 79% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine hydrogen iodide In acetonitrile for 6h; Heating; | 78% |
| With Amberlite IRA-400; borohydride form; copper(II) sulfate In methanol at 20℃; for 1h; Reduction; | 96 % Chromat. |
| With Graphite; benzaldehyde; potassium bromide In water at 25℃; Electrolysis; | 10 %Chromat. |

| Conditions | Yield |
|---|---|
| sulfuric acid Heating; | 71% |

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene at 100℃; for 0.05h; Temperature; Microwave irradiation; | 71% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether Mechanism; other propiolactones; | 70% |
| With potassium 18-crown-6 In tetrahydrofuran at -20℃; | 70% |
-
-
97-64-3, 2676-33-7
ethyl 2-hydroxypropionate

-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With hydrogen at 220℃; under 37503.8 Torr; for 12h; Catalytic behavior; | 68% |
| With hydrogen at 220℃; under 37503.8 Torr; for 12h; | 68% |
-
-
60-29-7
diethyl ether

-
-
201230-82-2
carbon monoxide

-
-
74-88-4
methyl iodide

-
A
-
74-96-4
ethyl bromide

-
B
-
141-78-6
ethyl acetate

-
C
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| 1,5-hexadienerhodium(I)-chloride dimer; potassium iodide at 150℃; under 14710.2 Torr; | A n/a B 42 % Chromat. C 60% |

-
-
802294-64-0
propionic acid

-
-
298-06-6
O,O-Diethyl hydrogen phosphorodithioate

-
A
-
998-79-8
ethyl dithiopropionate

-
B
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| at 200℃; for 0.8h; | A 20% B 54% |

-
-
97-64-3, 2676-33-7
ethyl 2-hydroxypropionate

-
-
616-14-8
1-iodo-2-methyl-butane

-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| 52% |
-
-
36749-09-4
3,3-diethoxypentane

-
A
-
78-09-1
orthocarbonic acid tetraethyl ester

-
B
-
1128-76-3
3-chloro-benzoic acid ethyl ester

-
C
-
105-37-3
Ethyl propionate

-
D
-
105-58-8
Diethyl carbonate

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 15 - 30℃; for 0.5h; Further byproducts given; | A 18% B n/a C n/a D 50% |

-
-
27374-25-0
[(1-Ethoxycyclopropyl)oxy]trimethylsilane

-
A
-
141-28-6
diethyl adipate

-
B
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate; calcium carbonate In ethanol for 0.1h; Mechanism; Product distribution; Ambient temperature; other reaction partners, other solvents; oxidation and oxidative tandem additions to alkene and cycloalkenones; | A 7% B 48% |
-
-
64-17-5
ethanol

-
-
27374-25-0
[(1-Ethoxycyclopropyl)oxy]trimethylsilane

-
-
109-92-2
ethyl vinyl ether

-
A
-
19790-76-2
ethyl 5,5-diethoxypentanoate

-
B
-
141-28-6
diethyl adipate

-
C
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate; calcium carbonate Ambient temperature; | A 26% B 8% C 48% |

-
-
64-17-5
ethanol

-
-
109-92-2
ethyl vinyl ether

-
A
-
19790-76-2
ethyl 5,5-diethoxypentanoate

-
B
-
141-28-6
diethyl adipate

-
C
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With calcium carbonate Ambient temperature; other reaction partners, other solvents; dimerization, reaction with solvent, addition to alkene and cycloalkenones; | A 26% B 8% C 48% |

-
-
10170-69-1
dimanganese decacarbonyl

-
-
535-11-5, 41978-69-2
Ethyl 2-bromopropionate

-
A
-
32884-97-2
diethyl 2,3-dimethylsuccinate

-
B
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| In chloroform-d1 Irradiation (UV/VIS); (N2 or Ar); 250-W sun lamp, 3 h; colorless solution is filtered, (1)H-NMR; | A 47% B 21% |

-
-
14516-54-2
bromopentacarbonylmanganese(I)

-
-
97-94-9
triethyl borane

-
-
917-58-8
potassium ethoxide

-
A
-
105-37-3
Ethyl propionate

-
B
-
96-22-0
pentan-3-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 16h; Sealed tube; | A 22% B 17% |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
34557-54-5
methane

-
A
-
105-37-3
Ethyl propionate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With F27-Tp(4Bo,3CF2CF3)Ag(thf) In carbon dioxide at 40℃; under 190013 Torr; for 14h; Supercritical conditions; | A 19% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With [hydrotris(3-trifluoromethyl-4,5,6,7-tetrafluoroindazolyl)borate]Ag(acetone) at 40℃; under 190013 Torr; for 14h; Catalytic behavior; Supercritical conditions; | 7% |
| With sodium dodecyl-sulfate In water at 20℃; under 121608 Torr; for 14h; |

| Conditions | Yield |
|---|---|
| Geschwindigkeit der Spaltung; |

-
-
67-56-1
methanol

-
-
121811-29-8, 138752-66-6, 138752-69-9, 759-66-0
ethyl 2-methyl-3-oxopentanoate

-
-
124-41-4
sodium methylate

-
A
-
554-12-1
propanoic acid methyl ester

-
B
-
105-37-3
Ethyl propionate


| Conditions | Yield |
|---|---|
| at 100 - 300℃; |
-
-
88-12-0
1-ethenyl-2-pyrrolidinone

-
-
105-37-3
Ethyl propionate

-
-
350017-26-4
3-propionyl-1-vinyl-2-pyrrolidone

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 3.7h; Heating / reflux; | 100% |

| Conditions | Yield |
|---|---|
| With potassium 2-methylbutan-2-olate In tetrahydrofuran at 20℃; for 11h; | 99% |
| Stage #1: acetonitrile With n-butyllithium In hexane at -78℃; for 1h; Stage #2: Ethyl propionate In hexane at -78 - -45℃; for 2h; Stage #3: With hydrogenchloride In hexane; water pH=2; | 80% |
| Stage #1: acetonitrile With n-butyllithium In hexane at -78℃; for 1h; Stage #2: Ethyl propionate In hexane at -78 - -45℃; for 2h; Stage #3: With hydrogenchloride; water In hexane pH=2; | 80% |
-
-
105-37-3
Ethyl propionate

-
-
6065-82-3
Ethyl diethoxyacetate

-
-
24132-51-2
ethyl 4,4-diethoxy-2-methyl-3-oxobutanoate

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78 - 20℃; Claisen condensation; | 99% |
| With sodium at 80℃; | |
| With sodium ethanolate |

| Conditions | Yield |
|---|---|
| With potassium 2-methylbutan-2-olate In tetrahydrofuran at 20℃; for 0.333333h; | 99% |
| Stage #1: Ethyl propionate With potassium tert-butylate In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: phenylacetonitrile In tetrahydrofuran at 20℃; | 80% |
| With potassium 2-methylbutan-2-olate In tetrahydrofuran at 20℃; for 0.5h; | 28% |

-
-
105-37-3
Ethyl propionate

-
-
56-35-9
bis(tri-n-butyltin)oxide

-
-
2207-46-7
3-tert-butyl-isoxazole-5-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In toluene | 97% |
| In toluene | 97% |

-
-
105-37-3
Ethyl propionate

-
-
623-70-1
ethyl (E)-crotonate

-
-
344883-66-5
Diethyl 2,3-dimethylpentanedioate

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; for 1h; | 96% |
-
-
106105-29-7
2-amino-4,5-dimethyl-1-phenyl-1H-pyrrole-3-carbonamide

-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 6h; Heating; | 96% |
| With sodium ethanolate In ethanol at 80℃; under 15001.5 Torr; for 30h; Inert atmosphere; | 94% |

-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With Trimethylacetic acid In ortho-propionate | 96% |
-
-
105-37-3
Ethyl propionate

| Conditions | Yield |
|---|---|
| With dmap; disodium mercaptoundecahydrododecaborate In acetonitrile for 24h; | 96% |
-
-
105-37-3
Ethyl propionate

-
-
508191-77-3
3-phenylsydnone

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; triethanolamine; ascorbic acid sodium salt In water; tert-butyl alcohol at 60℃; for 16h; | 96% |
-
-
105-37-3
Ethyl propionate

-
-
802294-64-0
propionic acid

| Conditions | Yield |
|---|---|
| With hydrazine In ethanol | 95% |
| With Candida antarctica lipase B; 4-nitro-phenol; MOPS buffer In water at 25℃; pH=7.2; Enzyme kinetics; Further Variations:; Reagents; Enzymatic reaction; | |
| With ethanol; sodium hydroxide for 1h; Reflux; |

| Conditions | Yield |
|---|---|
| Stage #1: Ethyl propionate With N,N-diethyl-N-isopropylamine; di-n-butylboryl trifluoromethanesulfonate In dichloromethane at -70℃; for 3.5h; Stage #2: acrolein In dichloromethane at -78 - 0℃; for 5h; | 95% |
-
-
70978-37-9
1-(azidomethyl)-4-methoxybenzene

-
-
105-37-3
Ethyl propionate

-
-
81581-05-7
ethyl 1-(4-methoxybenzyl)-1H-1,2,3-triazole-4-carboxylate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 0 - 20℃; | 95% |

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 16h; | 94% |
-
-
20808-12-2
fluoromethyl phenyl sulfone

-
-
105-37-3
Ethyl propionate

-
-
1151549-57-3
1-fluoro-1-(phenylsulfonyl)butan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: fluoromethyl phenyl sulfone; Ethyl propionate With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water at -78℃; Inert atmosphere; | 93% |
-
-
1570-45-2
isonicotinic acid ethylester

-
-
105-37-3
Ethyl propionate

-
-
66269-84-9
ethyl 2-methyl-3-oxo-3-(pyridin-4-yl)propanoate

| Conditions | Yield |
|---|---|
| Stage #1: isonicotinic acid ethylester; Ethyl propionate In tetrahydrofuran at -40℃; for 0.0833333h; Claisen Condensation; Inert atmosphere; Stage #2: With lithium hexamethyldisilazane In tetrahydrofuran at -40 - 20℃; for 0.833333h; Claisen Condensation; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxyphenylacetylen With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: Ethyl propionate With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -78℃; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: benzyl bromide With magnesium; lithium chloride In tetrahydrofuran at 63 - 67℃; Stage #2: Ethyl propionate In tetrahydrofuran at 30 - 40℃; for 1.5h; Time; | 92.4% |
Ethyl propionate Consensus Reports
Reported in EPA TSCA Inventory.
Ethyl propionate Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
Ethyl propionate Specification
The IUPAC name of this chemical is Ethyl propionate. With the CAS registry number 105-37-3 and EINECS registry number 203-291-4, it is also named as propanoic acid, ethyl ester. In addition, the molecular formula is C5H10O2. It is the ethyl ester of propionic acidand. It is used for various natural and synthetic resin solvents. Besides, it is also used for synthetic organic intermediates in the production of antimalarial medicine.
Physical properties about this chemical are: (1)ACD/LogP: 1.24; (2)ACD/LogD (pH 5.5): 1.24; (3)ACD/LogD (pH 7.4): 1.24; (4)ACD/BCF (pH 5.5): 5.15; (5)ACD/BCF (pH 7.4): 5.15; (6)ACD/KOC (pH 5.5): 112.56; (7)ACD/KOC (pH 7.4): 112.56; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.387; (12)Molar Refractivity: 26.98 cm3; (13)Molar Volume: 114.5 cm3; (14)Polarizability: 10.69 ×10-24cm3; (15)Surface Tension: 24.8 dyne/cm; (16)Density: 0.891 g/cm3; (17)Flash Point: 12.2 °C; (18)Enthalpy of Vaporization: 33.55 kJ/mol; (19)Boiling Point: 95.9 °C at 760 mmHg; (20)Vapour Pressure: 44.5 mmHg at 25°C.
Preparation of Ethyl propionate: it can be prepared by ethanol and propanoic acid. Add ethanol and propanoic acid into the reactor at first. Then add calcium chloride into the mixture with stirring. This reaction should reflux for 10 hours by heating and stirring. And then after a series of distillation, washing by sodium carbonate solution, drying and distillation again you can get the desired product.
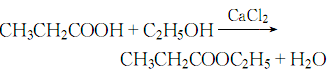
Uses of Ethyl propionate: it can be used for food flavoring agents. And it can react with ethane-1,2-diol to get propionic acid-(2-hydroxy-ethyl ester) and 1,2-bis-propionyloxy-ethane. This reaction will need catalyst Dowex 50W and solvent octane. The reaction time is 4 hours at reaction temperature of 100 °C. The yield is about 81%.
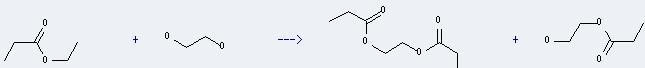
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable. During using it, you should not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer). And you should avoid contact with skin. In addition, you can keep away from sources of ignition - No smoking. Moreover, you can not empty it into drains. You should take precautionary measures against static discharges.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)CC
(2)InChI: InChI=1/C5H10O2/c1-3-5(6)7-4-2/h3-4H2,1-2H3
(3)InChIKey: FKRCODPIKNYEAC-UHFFFAOYAW
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1158mg/kg (1158mg/kg) | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 18, Pg. 109, 1960. | |
| rabbit | LDLo | skin | 14256mg/kg (14256mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 174, 1992. |
| rat | LD50 | intraperitoneal | 1200mg/kg (1200mg/kg) | Food and Cosmetics Toxicology. Vol. 16, Pg. 749, 1978. | |
| rat | LD50 | oral | 8732mg/kg (8732mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES GASTROINTESTINAL: OTHER CHANGES | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 1, Pg. 174, 1992. |
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 10537-47-0
- 10537-63-0
- 105379-24-6
- 105382-09-0
- 105384-38-1
- 10538-48-4
- 10538-49-5
- 10538-51-9
- 105388-21-4
- 105389-36-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View