-
Name
Ethofenprox
- EINECS 407-980-2
- CAS No. 80844-07-1
- Article Data8
- CAS DataBase
- Density 1.1±0.1 g/cm3
- Solubility <0.001 mg l-1(25 °C)
- Melting Point 36oC
- Formula C25H28O3
- Boiling Point 481.6±40.0 °C at 760 mmHg
- Molecular Weight 376.496
- Flash Point 165.1±24.6 °C
- Transport Information
- Appearance white solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-(4-Ethoxyphenyl)-2-methylpropyl3-phenoxybenzyl ether;4-Ethoxyneophyl 3-phenoxybenzyl ether;Ethophenprox;Ethoproxyfen;Ethoproxyphen;Etof;Etofenprox;MTI 500;SA130301;Trebon;Ethofenprox;
- PSA 27.69000
- LogP 6.37200
Synthetic route
-
-
51632-16-7
3-phenoxybenzyl bromide

-
-
83493-63-4
2-(4-ethoxyphenyl)-2,2-dimethylethanol

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran for 0.583333h; Irradiation; | 95% |

-
-
83493-63-4
2-(4-ethoxyphenyl)-2,2-dimethylethanol

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| at 50℃; for 2h; | 93% |
-
-
75-03-6
ethyl iodide

-
-
80854-21-3
3-phenoxybenzyl 2-(4-hydroxyphenyl)-2-methylpropyl ether

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 25℃; for 14h; Inert atmosphere; | 86% |
-
-
13826-35-2
3-Phenoxybenzyl alcohol

-
-
83493-80-5
4-(2'-chloro-1',1'-dimethylethyl)ethoxybenzene

-
A
-
80844-07-1
etofenprox

-
B
-
80854-21-3
3-phenoxybenzyl 2-(4-hydroxyphenyl)-2-methylpropyl ether

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 110 - 120℃; for 15h; | A 42.5% B 8.5% |

-
-
51632-16-7
3-phenoxybenzyl bromide

-
A
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran for 8h; |

-
-
515-40-2
neophyl chloride

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 91 percent / HNO3; H2SO4 2: 67 percent / K2CO3 / acetone / 15 h / 40 °C 3: 42.5 percent / KOH / dimethylsulfoxide / 15 h / 110 - 120 °C View Scheme |
-
-
99359-77-0
1-chloro-2-methyl-2-(4-nitrophenyl)propane

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 67 percent / K2CO3 / acetone / 15 h / 40 °C 2: 42.5 percent / KOH / dimethylsulfoxide / 15 h / 110 - 120 °C View Scheme |
-
-
103-73-1
Phenetole

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2SO4 / 5 h / 20 °C 2: 42.5 percent / KOH / dimethylsulfoxide / 15 h / 110 - 120 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / H2SO4 2: 91 percent / HNO3; H2SO4 3: 67 percent / K2CO3 / acetone / 15 h / 40 °C 4: 42.5 percent / KOH / dimethylsulfoxide / 15 h / 110 - 120 °C View Scheme |
-
-
57225-86-2
methyl 2-(4-chlorophenyl)-2-methylpropanoate

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / 2 h / 0 - 20 °C / Inert atmosphere 2: sodium hydride / N,N-dimethyl-formamide / 2 h / 0 - 20 °C / Inert atmosphere 3: potassium hydroxide; tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos / water; 1,4-dioxane / 16 h / 100 - 115 °C / Inert atmosphere 4: potassium carbonate / N,N-dimethyl-formamide / 14 h / 0 - 25 °C / Inert atmosphere View Scheme |
-
-
80854-14-4
2-(4-chlorophenyl)-2-methylpropyl alcohol

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydride / N,N-dimethyl-formamide / 2 h / 0 - 20 °C / Inert atmosphere 2: potassium hydroxide; tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos / water; 1,4-dioxane / 16 h / 100 - 115 °C / Inert atmosphere 3: potassium carbonate / N,N-dimethyl-formamide / 14 h / 0 - 25 °C / Inert atmosphere View Scheme |

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium hydroxide; tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos / water; 1,4-dioxane / 16 h / 100 - 115 °C / Inert atmosphere 2: potassium carbonate / N,N-dimethyl-formamide / 14 h / 0 - 25 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With Thianthrene In acetonitrile at -78 - 25℃; | 84% |


| Conditions | Yield |
|---|---|
| Stage #1: thianthrene-5-oxide; etofenprox With trifluoroacetic anhydride In acetonitrile at -78 - 25℃; Sealed tube; Stage #2: sodium tetrafluoroborate In dichloromethane; water | 84% |

-
-
39082-53-6
1,1-bis(phenylsulfonyl)ethylene

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| With [Ir(3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl)2(4,4'-bis(trifluoromethyl)bipyridine)]PF6 In dichloromethane for 48h; Sealed tube; Inert atmosphere; Irradiation; | 84% |
-
-
67969-82-8
tetrafluoroboric acid diethyl ether

-
-
13755-29-8
sodium tetrafluoroborate

-
-
2362-50-7
thianthrene-5-oxide

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Stage #1: tetrafluoroboric acid diethyl ether; thianthrene-5-oxide; etofenprox With trifluoroacetic anhydride In acetonitrile at -40 - 25℃; for 15h; Schlenk technique; Stage #2: sodium tetrafluoroborate In dichloromethane; water | 74% |


| Conditions | Yield |
|---|---|
| With iodosodilactone at 90℃; for 18h; Sealed tube; | 42% |
-
-
25370-97-2
O-(p-toluenesulfonyl)-N-methylhydroxylamine

-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| With air at 20℃; for 36h; chemoselective reaction; | 37% |
-
-
80844-07-1
etofenprox

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trifluoroacetic anhydride / acetonitrile / 15 h / -40 - 25 °C / Schlenk technique 2: tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate; tetrakis(acetonitrile)copper(I)tetrafluoroborate; tetramethyl ammoniumhydroxide / acetonitrile; dimethyl sulfoxide / 8 h / 15 °C / Inert atmosphere; Irradiation View Scheme |
Etofenprox Specification
The Etofenprox, with the CAS registry number 80844-07-1, is also known as 4-Ethoxyneophyl 3-phenoxybenzyl ether. Its EINECS number is 407-980-2. This chemical's molecular formula is C25H28O3 and molecular weight is 376.49. What's more, its systematic name is 1-{[2-(4-Ethoxyphenyl)-2-methylpropoxy]methyl}-3-phenoxybenzene. This chemical should be sealed and stored in a cool and dry place. It is incompatible with strong oxidizing agents. Its classification codes are: (1)Acaricide; (2)Agricultural Chemical; (3)Insecticide; (4)Reproductive Effect. This chemical is used as a pesticide of broad-spectrum, high effective, low toxic, less residual and it is safe to crop. It is synthetic pyrethroid for insecticidal efficacy against mosquito larvae & on non-target organisms.
Physical properties of Etofenprox are: (1)ACD/LogP: 7.34±0.52 # of Rule of 5 Violations: 1; (2)ACD/LogD (pH 5.5): 7.35 ACD/LogD (pH 7.4): 7.35; (3)ACD/BCF (pH 5.5): 225191.00; (4)ACD/BCF (pH 7.4): 225191.00; (5)ACD/KOC (pH 5.5): 236010.60; (6)ACD/KOC (pH 7.4): 236010.60; (7)#H bond acceptors: 3; (8)#H bond donors: 0; (9)#Freely Rotating Bonds: 9; (10)Polar Surface Area: 27.69 Å2; (11)Index of Refraction: 1.559; (12)Molar Refractivity: 113.3±0.3 cm3; (13)Molar Volume: 350.8±3.0 cm3; (14)Polarizability: 44.9±0.5×10-24cm3; (15)Surface Tension: 38.6±3.0 dyne/cm; (16)Density: 1.1±0.1 g/cm3; (17)Flash Point: 165.1±24.6 °C; (18)Enthalpy of Vaporization: 71.8±3.0 kJ/mol; (19)Boiling Point: 481.6±40.0 °C at 760 mmHg; (20)Vapour Pressure: 0.0±1.2 mmHg at 25°C.
Preparation of Etofenprox: this chemical can be prepared by 1-(bromomethyl)-3-phenoxybenzene and tetrahydrofuran by irradiation. This reaction time is 35 min. The yield is about 95%.
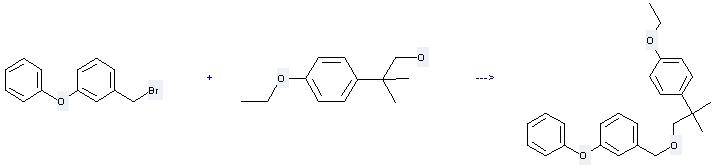
You can still convert the following datas into molecular structure:
(1)SMILES: CCOC1=CC=C(C=C1)C(C)(C)COCC2=CC(=CC=C2)OC3=CC=CC=C3
(2)Std. InChI: InChI=1S/C25H28O3/c1-4-27-22-15-13-21(14-16-22)25(2,3)19-26-18-20-9-8-12-24(17-20)28-23-10-6-5-7-11-23/h5-17H,4,18-19H2,1-3H3
(3)Std. InChIKey: YREQHYQNNWYQCJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 5gm/kg (5000mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| duck | LD50 | unreported | > 2gm/kg (2000mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| mouse | LD50 | intraperitoneal | > 13400mg/kg (13400mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| mouse | LD50 | oral | > 107gm/kg (107000mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| mouse | LD50 | skin | > 2140mg/kg (2140mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| mouse | LD50 | subcutaneous | > 53600mg/kg (53600mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| rat | LC50 | inhalation | > 5900mg/m3/4H (5900mg/m3) | Nippon Noyaku Gakkaishi. Journal of the Pesticide Science Society of Japan. Vol. 15, Pg. 505, 1989. | |
| rat | LD50 | intraperitoneal | > 42880mg/kg (42880mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| rat | LD50 | oral | > 42800mg/kg (42800mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| rat | LD50 | skin | > 2140mg/kg (2140mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. | |
| rat | LD50 | subcutaneous | > 32160mg/kg (32160mg/kg) | Japan Pesticide Information. Vol. (48), Pg. 23, 1986. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View