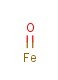Synthetic route
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| With water; hydrogen In neat (no solvent) byproducts: Fe; formation of FeO at 800°C;; | 92% |
| With water; hydrogen In neat (no solvent) byproducts: Fe; formation of FeO at 700°C;; | 85% |
| With hydrogen In neat (no solvent) redn. of Fe2O3 in a stream of H2 below red heat;; | |
-
-
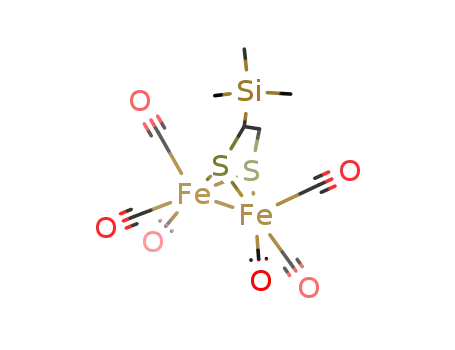
1-trimethylsilyl-μ3-S,S'-ethylenedithiolatohexacarbonyldiiron
-
B
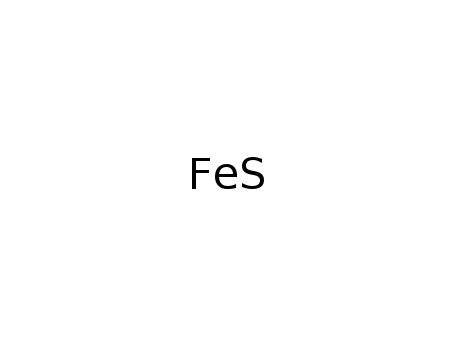
-
iron sulfide
-
C
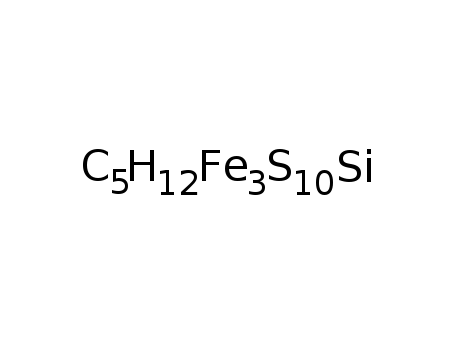
-
(CH3)3SiC2H3S10Fe3
Conditions
| Conditions | Yield |
|---|
| In decane byproducts: Me3SiCHCH2, CH2CH2, CH3CHCH2; O2 atmosphere; decompn. (170°C, 8 h); further byproducts: (Me3Si)2O, (Me2SiO)3 and (Me2SiO)4; GLC, chromato-mass spectroscopy; | A 20%
B 70%
C 10% |
-
-
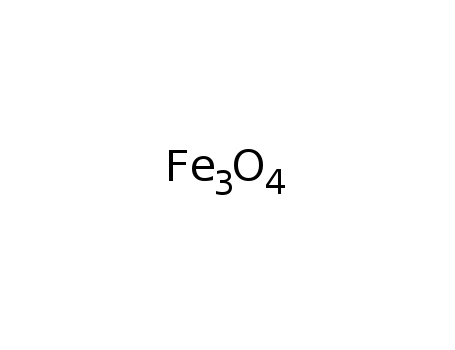
iron(II,III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) formation of FeO during 24 h at 600°C;; | 51% |
| In neat (no solvent) formation of FeO during 24 h at 592°C;; | 24% |
| In neat (no solvent) preparation of FeO in the high-frequenzy induction furnace;; | |
-
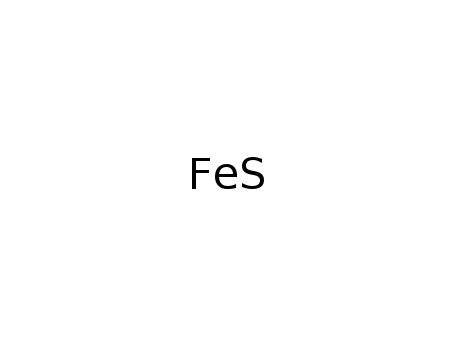
-
iron sulfide
-

-
calcium oxide
-
B

-
iron calcium oxysulfide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) pressed into pellets, fired at 1190 K for 4 h in flowing He; obtained as a mixt.; | A 1%
B n/a |
-
A

-
iron(II,III) oxide
-
C

-
iron(III) oxide
-
D
-
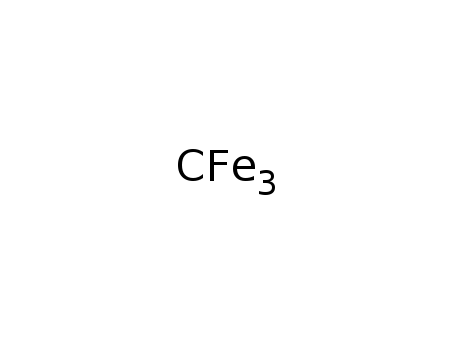
cementite
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, gas phase) mixt. of vapor of Fe(CO)5 and H2 decomposed by plasma-chemical decomposition on Al2O3; monitored by XRD; | A n/a
B n/a
C n/a
D 1%
E n/a |
-
-
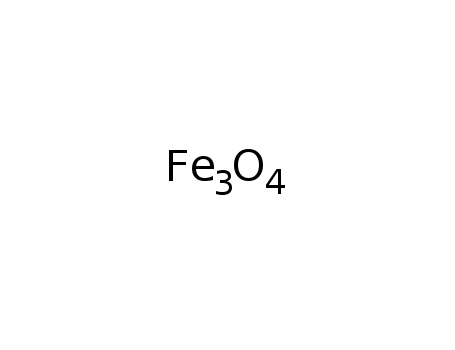
iron oxide
-

-
iron(II) titanate
-
B

-
Fe2.40Ti0.60O4
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) ball milled Fe2TiO4 was mixed with stoich. amts. of Fe3O4; ball milled for 10 min; placed in steel crucible; heated at 950°C for 60 h under Ar; cooled in furnace under Ar atm. to 400°C; air cooled to room temp.; identified by X-ray diffraction; | A 1%
B n/a |
-
B

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In N,N-dimethyl-formamide at 160℃; for 2h; Heating / reflux; | |
-
B
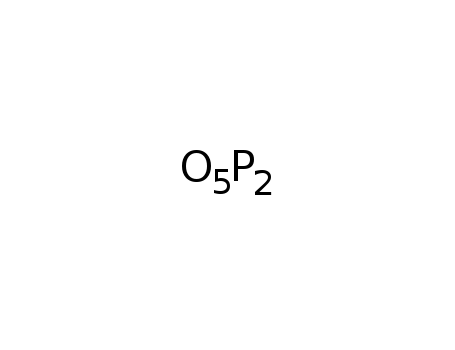
-
phosphorus pentoxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) the equilibrium constant was determined;; | |
-

-
iron(II) metasilicate
Conditions
| Conditions | Yield |
|---|
| With methyllithium In neat (no solvent) byproducts: SiO2; heating iron(II)-silicate for 1 h at 1200°C in presence of excess of lime; dissociation in FeO and SiO2;; | >99 |
-

-
iron(III) oxide
-
A

-
iron(II,III) oxide
Conditions
| Conditions | Yield |
|---|
| With carbon In neat (no solvent, solid phase) byproducts: CO, CO2; under Ar, mixt. of Fe2O3 and C heated at 800°C for 1 h; identified by X-ray analysis; | |
-

-
iron(III) oxide
-
A

-
iron(II,III) oxide
Conditions
| Conditions | Yield |
|---|
| With H2 or CO In neat (no solvent) redn. of Fe2O3 in a stream of H2 or CO; first formation of Fe3O4; at higher temp. formation of FeO and Fe;; | |
| With H2 or CO In neat (no solvent) redn. of Fe2O3 in a stream of H2 or CO; first formation of Fe3O4; at higher temp. formation of FeO and Fe;; | |
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| With carbon dioxide; carbon monoxide In neat (no solvent) byproducts: C; redn. of at 400°C calcined Fe2O3 starts at 200-230°C; with begin of FeO-formation starts pptn. of carbon; pptn. of carbon ends with formation of Fe;; | |
| In neat (no solvent) redn. of Fe2O3 in carbon to FeO and Fe below 100°C;; | |
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| dissocn. of Fe2O3 in glass to O2 and FeO about 1300°C;; | |
| decompn. of Fe2O3 to FeO and oxygen;; | |
| dissocn. of Fe2O3 in glass to O2 and FeO about 1300°C;; | |
| decompn. of Fe2O3 to FeO and oxygen;; | |
-
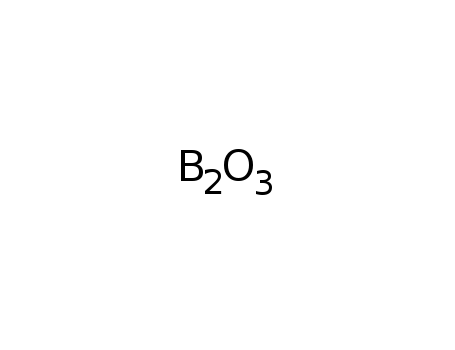
-
boron trioxide
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In melt byproducts: 3B2O3*2FeO*2Fe2O3;; melting in the air for a long period of time;; | |
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| exactly controlled H2O/H2 ratio, sufficiently high flow rate of gas mixt., use of lowly sintered well powdered Fe2O3; | |
-
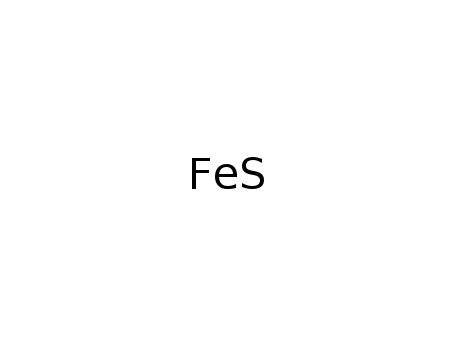
-
iron sulfide
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: Fe3O4; heating of an oxide-sulfide-mixture in an N2-stream;; no formation of FeO below 900°C;; | A 0%
B n/a |
-

-
iron(III) oxide
-

-
zinc sulfide
-
C

-
zinc(II) oxide
Conditions
| Conditions | Yield |
|---|
| direct smelting of sulfide ores; unreduced Fe2O3 roasts zinc blende;; | |
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) neutral atmosphere; 1300°C; | |
| In melt equilibrium constant was determined;; | |
| In neat (no solvent) complete reaction at elevated temps.;; | |
-

-
iron(III) oxide
-
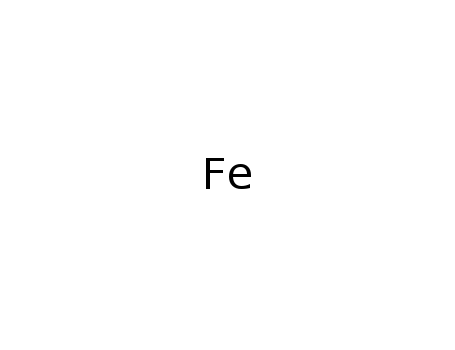
-
iron
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) powders of Fe2O3 + αFe (25 or 50%) were treated in a planetary high-energy ball mill for up to 3 h; powder XRD; Moessbauer spectra; | |
-

-
iron(III) oxide
-

-
iron(III) oxide
-
A
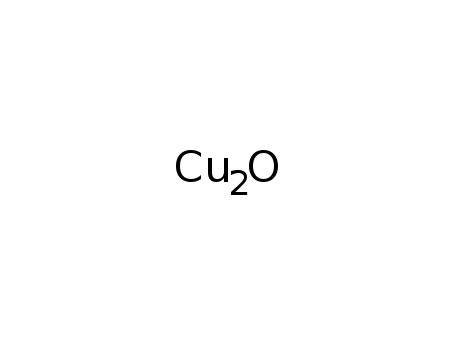
-
copper(I) oxide
-
C
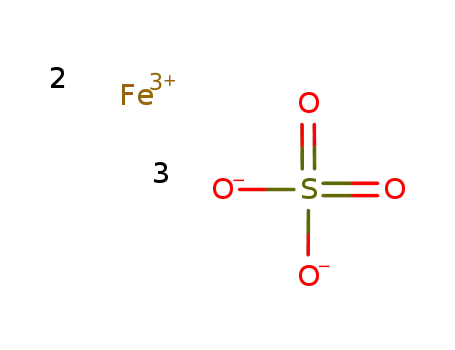
-
iron(III) sulfate
-
D
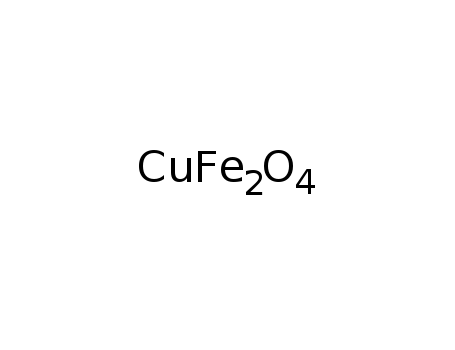
-
copper(II) ferrite
-
E

-
copper(II) oxide
Conditions
| Conditions | Yield |
|---|
| With air byproducts: SO2, SO3; mixing of Fe2O3 and Cu2S (1:1) mech. before roasting; information about the react. eqs. in detail, about dependence on temp. from 300 till 550°C and about the effect of Fe2O3; further products; | |
| With air byproducts: SO2, SO3; mixing of Fe2O3 and Cu2S (1:1) mech. before roasting; information about the react. eqs. in detail, about dependence on temp. from 300 till 550°C and about the effect of Fe2O3; further products; | |
-

-
iron(III) oxide
-
A

-
copper(I) oxide
-
C

-
copper(II) ferrite
-
D

-
copper(II) oxide
Conditions
| Conditions | Yield |
|---|
| byproducts: SO2, SO3; at roasting of Cu2S in presence of Fe2O3; | |
-
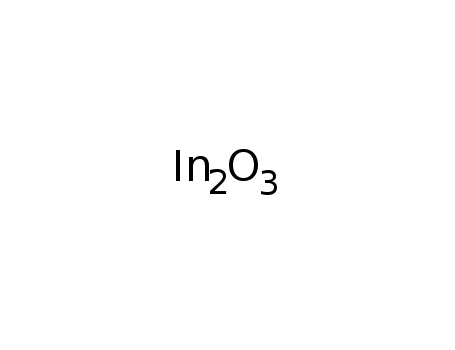
-
indium(III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) (Ar); In2O3 and Fe reacted in 1:1 or 1:2 or 2:1 molar ratio; powdered inmortar; sealed under vacuum in quartz ampoule; heated to 723 K and with 0.5 K/h to 973 K; maintained for 7 days; cooled to 0.8 K/h to 473 K; le ft standing at room temp.; | |
-
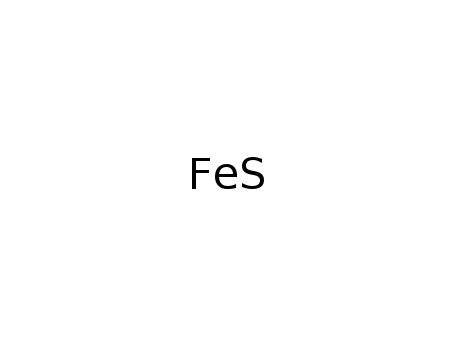
-
iron sulfide
-
B

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: H2; thin layer of FeO (71.5%) and Fe2O3 (26.34%) is formed inside a steam pipe; better reaction in the presence of O2 or CO2, formation of some H2;; | |
| In neat (no solvent) byproducts: H2; thin layer of FeO (71.5%) and Fe2O3 (26.34%) is formed inside a steam pipe; better reaction in the presence of O2 or CO2, formation of some H2;; | |
-
A

-
iron(II,III) oxide
-
C

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) react. of Fe with water vapor at 700°C; cooling on air;; radiographic anal.;; | |
Conditions
| Conditions | Yield |
|---|
| With oxygen In neat (no solvent) byproducts: Fe2O3, Fe3O4; iron foil was treated with a steam/N2 mixt. with added air (0.13-1.44% O2) at 800°C for 1 h; product samples (thin films) were investigated by gravimetric anal., Auger and X-ray spectroscopy, scanning electron microscopy; | |
| In neat (no solvent) byproducts: Fe3O4; iron foil was treated with steam/N2 mixts. at 540-800°C for 3-17h; growth rate and composition of the product depend on the foil thickness, H2O flow rate, reaction time and temp.; product samples (thin films) were investigated by gravimetric anal., Auger and X-ray spectroscopy, scanning electron microscopy; | |
| In neat (no solvent) byproducts: H2; oxidation of Fe with water vapor above 500°C;; | |
Conditions
| Conditions | Yield |
|---|
| In water reaction of iron shavings with water at 100°C in air or N2 atmosphere;; | |
| H2O/H2 equil. ratio detd.; | |
| study of chemical equil.; | |
-

-
pyrite
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) heating pyrite in water vapor at 300-400°C;; | |
-
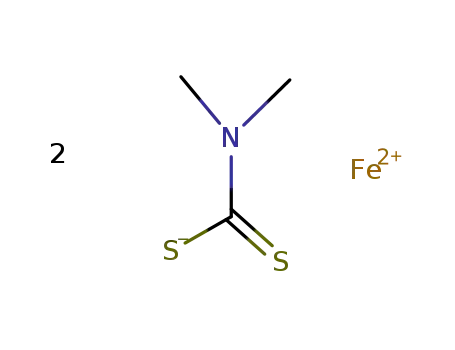
-
ferric dimethyldithiocarbamate
Conditions
| Conditions | Yield |
|---|
| With zinc(II) oxide In water at 20℃; for 8h; Reflux; Large scale; | 99% |
-
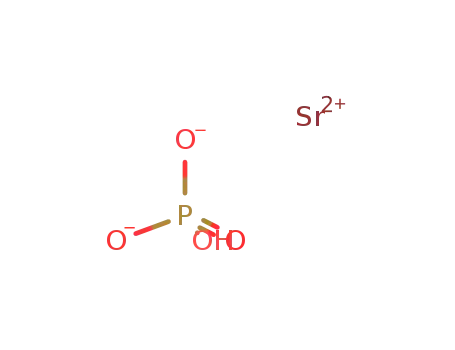
-
strontium hydrogenphosphate
-
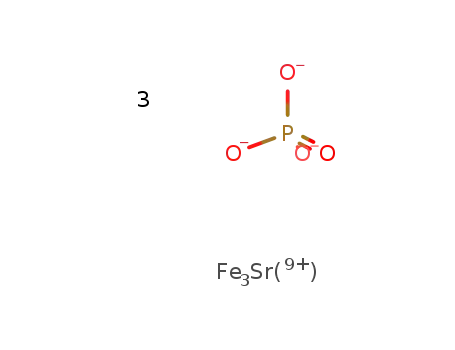
-
SrFe3(9+)*3PO4(3-)=SrFe3(PO4)3
Conditions
| Conditions | Yield |
|---|
| In water hydrothermal method; heating (sealed quartz tube, 648 K, 3 d), cooling (2 h to room temp.); filtration, washing (water, acetone), drying (335 K, 1 h); elem anal. (EDX), detn. by powder XRD; | 91% |
-

-
iron(III) oxide
-
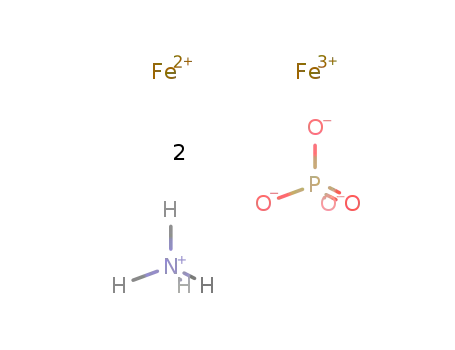
-
ammonium iron(II,III) phosphate
Conditions
| Conditions | Yield |
|---|
| In water sealed gold ampoule, 500°C (32000 psi autogenous pressure), 24 h;cooling to 250°C at 3°C/h, quenching to room temp.; | 85% |
-
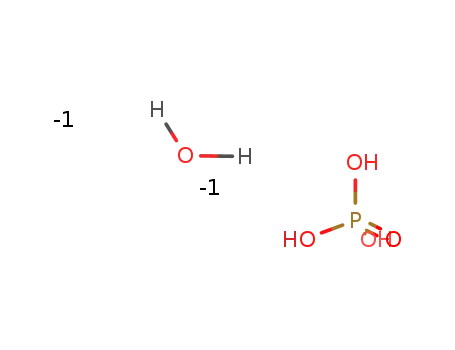
-
(x)H2O*(x)H3O4P
-
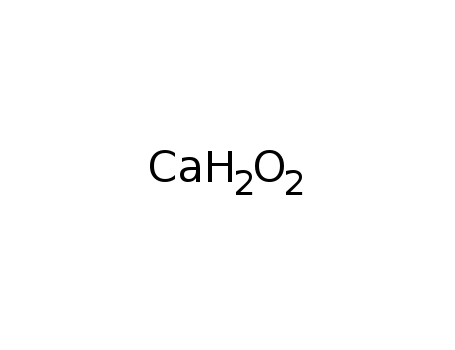
-
calcium hydroxide
-
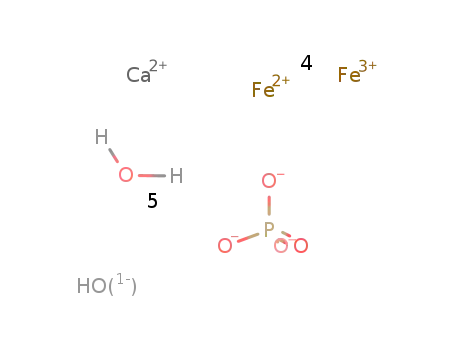
-
Ca(2+)*4Fe(3+)*Fe(2+)*5PO4(3-)*OH(1-)*H2O=CaFe5(PO4)5(OH)*H2O
Conditions
| Conditions | Yield |
|---|
| In further solvent(s) High Pressure; heating in autoclave, 400°C, 2.5 d; cooling to room temp. within 8h; detn. by X-ray powder diffraction; | 84% |
-
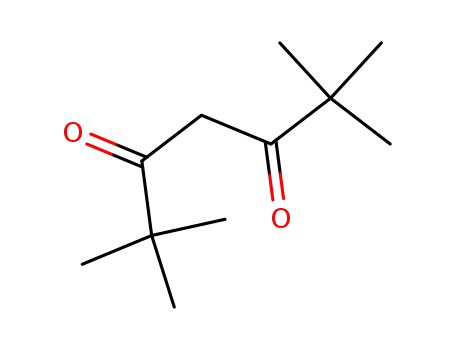
-
1118-71-4
2,2,6,6-tetramethylheptane-3,5-dione
-
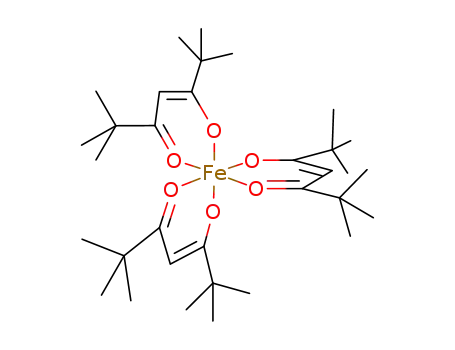
-
iron(III) 2,2,6,6-tetramethyl-3,5-heptadionate
Conditions
| Conditions | Yield |
|---|
| With O2 In further solvent(s) dione as solvent, reflux for 24 h under O2; cooling to room temp., evapn. in vac., extn. with acetone, evapn. at room temp. in air or in vac., sublimation, recrystn. from EtOH, elem. anal.; | 60% |
-
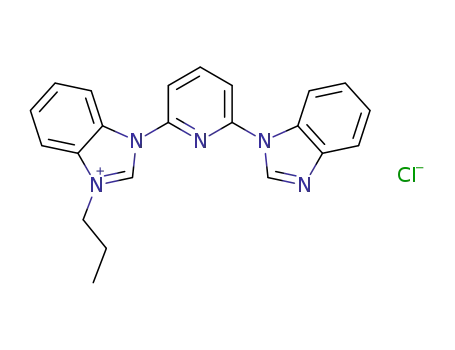
-
C22H20N5(1+)*Cl(1-)
-
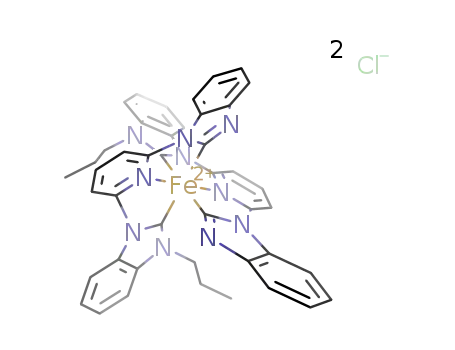
-
C44H36FeN10(2+)*2Cl(1-)
Conditions
| Conditions | Yield |
|---|
| In dimethyl sulfoxide at 110℃; under 7500.75 Torr; for 24h; Inert atmosphere; | 38% |
-

-
rubidium chloride
-
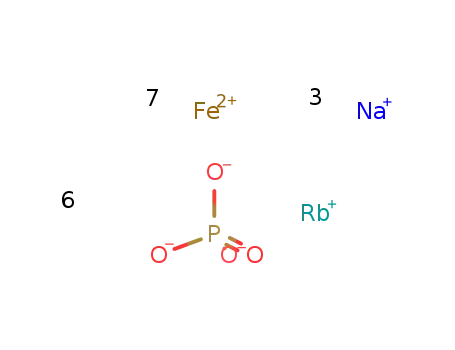
-
Rb(1+)*3Na(1+)*7Fe(2+)*6PO4(3-) = RbNa3Fe7(PO4)6
Conditions
| Conditions | Yield |
|---|
| In melt byproducts: (RbCl)Na2Fe3(P2O7)2, NaFe3.67(PO4)3; FeO and P4O10 ground in RbCl/NaCl flux and placed in carbon-coated fused-silica ampoule; sealed under vac.; heated to 700°C and held at this temp. for 4 d; slowly cooled to 450°C and finally cooled to room temp.; | 20% |
Conditions
| Conditions | Yield |
|---|
| With citric acid In water at 55 - 60℃; for 1h; | |
-
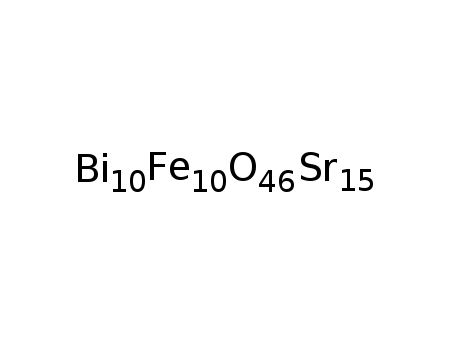
-
Bi10Sr15Fe10O46
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) alumina crucible, heating (1250°C), cooling (800°C, 105 h), annealing (765°C, 0.2% O2 or 60 atm O2), slow cooling (from 500to 350°C), holding (350 degree.C, 2 wk); | |
-

-
iron(III) oxide
-
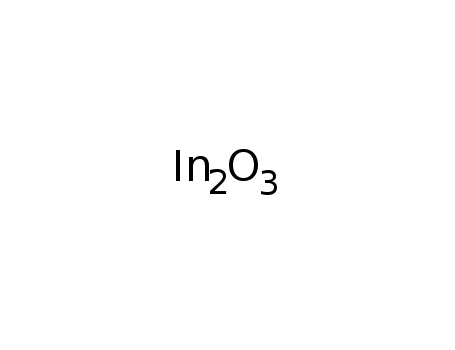
-
indium(III) oxide
-

-
zinc(II) oxide
-
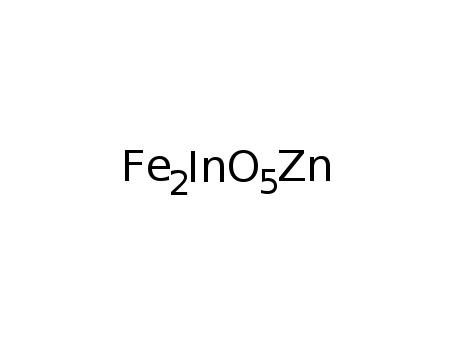
-
InFeO3(ZnO)(FeO)
Conditions
| Conditions | Yield |
|---|
| heating (sealed Pt tube, 1200°C, 1 d), rapid cooling in air; | |
-

-
iron(III) oxide
-
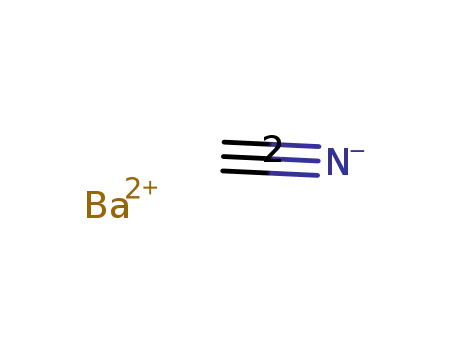
-
barium cyanide
-
B
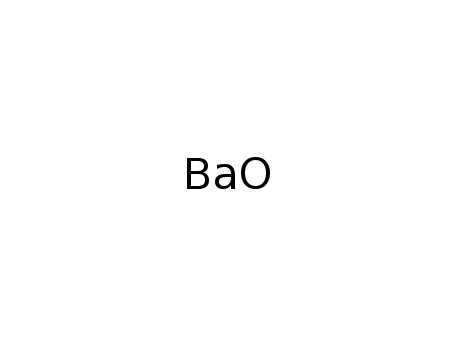
-
barium(II) oxide
-

-
iron(III) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Electrolysis; warming mixture of FeO and Fe2O3 with direct current (needed for electrolysis) in chamotte tube, pptn. of Fe occurs at 1700°C on cathode, current efficiency depends on temperature, but is very low in common;; | |
| In melt Electrolysis; electrolyzing melt of FeO, Fe2O3 and SiO2 at 1300°C with high current density and terminal voltage 1.7 to 2 V leads to pptn. of iron sponge;; | |
Ferrous oxide Chemical Properties
Product Name: Iron(II) oxide (CAS NO.1345-25-1)
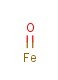
Molecular Formula: FeO
Molecular Weight: 71.84g/mol
Mol File: 1345-25-1.mol
EINECS: 215-721-8
Melting Point: 1369°C
Sensitive: Air Sensitive
Stability: Stable. Incompatible with strong oxidizing agents. Highly flammable.
H-Bond Donor: 0
H-Bond Acceptor: 1
Structure Descriptors of Iron(II) oxide (CAS NO.1345-25-1):
IUPAC Name: oxoiron
Canonical SMILES: O=[Fe]
InChI: InChI=1S/Fe.O
InChIKey: UQSXHKLRYXJYBZ-UHFFFAOYSA-N
Product Categories: Inorganic Chemicals; Inorganics; Metal and Ceramic Science; Oxides
Ferrous oxide Uses
Iron(II) oxide (CAS NO.1345-25-1) is used as a pigment. It is FDA-approved for use in cosmetics and it is used in some tattoo inks.
Ferrous oxide Production
Iron(II) oxide can be prepared by heating Ferrous oxide in vacuo:
FeC2O4 → FeO + CO + CO2
The black powder can be made less reactive by heating. The heated sample has a more physical change is quenched to prevent disproportionation.Stoichiometric FeO can be prepared by heating Fe0.95O with metallic iron at 770 °C and 36 kbar.
Ferrous oxide Consensus Reports
Related compounds: Iron(III) oxide, Iron(II,III) oxide
Ferrous oxide Safety Profile
Ignites when heated in air above 200°C. The powdered oxide may be pyrophoric. Incandescent or hazardous reaction with nitric acid (with powdered oxide), hydrogen peroxide, sulfur dioxide + heat. See also IRON and IRON COMPOUNDS.
Safety Information of Iron(II) oxide (CAS NO.1345-25-1):
Hazard Codes: F
Risk Statements: 11
R11:Highly flammable.
Safety Statements: 16-33-7/9
S16:Keep away from sources of ignition.
S33:Take precautionary measures against static discharges.
S7:Keep container tightly closed.
S9:Keep container in a well-ventilated place.
RIDADR: UN 3178 4.1/PG 2
Ferrous oxide Standards and Recommendations
DFG MAK: 1.5 mg/m3 calculated as fine dust.
Ferrous oxide Specification
Iron(II) oxide , its CAS NO. is 1345-25-1, the synonyms are Iron monooxide ; Natural wuestite ; Iron(II) oxide .
 F
F