-
Name
Guanidine hydrochloride
- EINECS 200-002-3
- CAS No. 50-01-1
- Article Data32
- CAS DataBase
- Density 1.18 g/mL at 25 °C(lit.)
- Solubility 2280 g/L (20 °C) in water
- Melting Point 180-185 °C(lit.)
- Formula CH5N3.HCl
- Boiling Point 132.9 °C at 760 mmHg
- Molecular Weight 95.5317
- Flash Point 34.2 °C
- Transport Information
- Appearance White cryst. powder
- Safety 26-36-22
- Risk Codes 22-36/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Guanidine,monohydrochloride (8CI,9CI);Buffer AL;Guanidine chloride;Guanidinehydrochloride;Guanidinium chloride;Guanidinium hydrochloride;
- PSA 75.89000
- LogP 1.14090
Synthetic route

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether at 0℃; Product distribution; | 78% |
-
-
1674-62-0
N1-methyl biguanide hydrochloride

-
A
-
420-04-2
CYANAMID

-
B
-
471-29-4
1-methylguanidine

-
C
-
50-01-1
guanidine hydrochloride

-
D
-
4674-68-4
methylcyanamide

| Conditions | Yield |
|---|---|
| bei der thermischen Zersetzung; |


| Conditions | Yield |
|---|---|
| With aluminium trichloride; ammonia at 300℃; |

-
A
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| In chloroform-d1 Equilibrium constant; pH 8; |

-
-
127099-85-8, 780722-26-1
N-Cyanoguanidine

-
A
-
645-93-2
ammelide

-
B
-
50-01-1
guanidine hydrochloride

-
C
-
56-03-1
BIGUANIDE

| Conditions | Yield |
|---|---|
| at 150℃; |

-
-
56-23-5
tetrachloromethane

-
-
7664-41-7
ammonia

-
-
7553-56-2
iodine

-
A
-
74-90-8
hydrogen cyanide

-
B
-
460-19-5
ethanedinitrile

-
C
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| at 140℃; |


| Conditions | Yield |
|---|---|
| at 100℃; |


-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With silica gel In melt 160 until 230°C; 1.5-fold excess of NH4Cl; in the presence of the same amt. of silica gel as urea; |
-
-
56-23-5
tetrachloromethane

-
B
-
127099-85-8, 780722-26-1
dicyandiamide

-
C
-
67-72-1
hexachloroethane

-
E
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With ammonia In tetrachloromethane other Radiation; γ-radiation of NH3 soln. in CCl4 at 25°C; mechanism discussed; further products and further intermediates;; |


| Conditions | Yield |
|---|---|
| 300°C; in autoclave; | |
| 300°C; in autoclave; |


| Conditions | Yield |
|---|---|
| With iodine; copper byproducts: HCN, cyanogen; High Pressure; 140°C; 17 h; | 35-40 |
| With Cu; I2 |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 4h; |
-
-
50-01-1
guanidine hydrochloride

-
-
94012-44-9, 94012-45-0
3-methylthio-1-phenyl-4-phenylsulfonyl-2-buten-1-one

-
-
106808-11-1
2-amino-4-phenyl-6-(phenylsulphonyl-methyl)pyrimidine

| Conditions | Yield |
|---|---|
| With sodium In ethanol for 2h; Heating; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
50-01-1
guanidine hydrochloride

-
-
219511-71-4
tert-butyl N-(diaminomethylidene)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrochloride With sodium hydroxide In water at 0℃; for 0.166667h; Inert atmosphere; Stage #2: di-tert-butyl dicarbonate In water; acetone at 0 - 20℃; for 2.5h; Inert atmosphere; | 100% |
| With sodium hydroxide In 1,4-dioxane for 15h; | 88% |
| With sodium hydroxide In 1,4-dioxane; water at 0 - 20℃; | 66% |
-
-
50-01-1
guanidine hydrochloride

-
-
126930-22-1
4-(Cyclohexyloxy)acetessigsaeure-ethylester

-
-
1161825-17-7
2-amino-6-(cyclohexyloxymethyl)pyrimidin-4-ol

| Conditions | Yield |
|---|---|
| With sodium methylate In ethanol Heating / reflux; | 100% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In 1,4-dioxane for 2h; Reflux; | 100% |
| With hydrazine hydrate In 1,4-dioxane for 2h; Reflux; Inert atmosphere; | 100% |
| With hydrazine hydrate In 1,4-dioxane for 2h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 16h; Solvent; Reagent/catalyst; Inert atmosphere; Reflux; | 100% |
-
-
2765-59-5
2-chloro-10-(3-chloropropyl)-10H-phenothiazine

-
-
50-01-1
guanidine hydrochloride

-
-
2518-14-1
[3-(2-chloro-phenothiazin-10-yl)-propyl]-guanidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 80℃; for 72h; | 100% |
-
-
50-01-1
guanidine hydrochloride

-
-
19227-70-4
guanidinium hydroiodide

| Conditions | Yield |
|---|---|
| With sodium iodide In methanol; acetone for 12h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With sodium methylate In ethanol at 90℃; for 16h; | 100% |
-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With silver(I) dinitramide In methanol | 99% |
| With dinitramide potassium salt In water at 20℃; for 0.5h; Green chemistry; | 3.3 g |
-
-
50-01-1
guanidine hydrochloride

-
-
60598-48-3
5-amino-1-benzyl-1H-imidazole-4-carbonitrile

-
-
7674-36-4
2,6-diamino-9-benzylpurine

| Conditions | Yield |
|---|---|
| In ethanol for 10h; Heating; | 99% |
-
-
22666-07-5
di-p-tolylbutadiyne

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With caesium carbonate; dimethyl sulfoxide at 120℃; for 12h; Sealed tube; Schlenk technique; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; dimethyl sulfoxide at 120℃; for 12h; Reagent/catalyst; Temperature; Sealed tube; Schlenk technique; regioselective reaction; | 99% |
| With caesium carbonate In dimethyl sulfoxide at 120℃; Reagent/catalyst; Sealed tube; | 99% |

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With caesium carbonate; dimethyl sulfoxide at 120℃; for 12h; Sealed tube; Schlenk technique; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In dimethyl sulfoxide at 120℃; Temperature; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 5 - 20℃; for 72h; | 99% |

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrochloride With potassium carbonate In ethanol at 20℃; for 0.666667h; Stage #2: 3-dimethylamino-1-(3-nitrophenyl)-2-propen-1-one In ethanol at 80℃; for 20h; | 98.7% |
-
-
50-01-1
guanidine hydrochloride

-
-
112-57-2
N-(2-aminoethyl)-N'-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine

-
-
638-53-9
tridecanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: N-(2-aminoethyl)-N'-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine; tridecanoic acid With toluene-4-sulfonic acid at 155℃; for 2.75h; Stage #2: guanidine hydrochloride at 152 - 176℃; for 5h; | 98.5% |


-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran Inert atmosphere; Reflux; | 98.5% |
-
-
50-01-1
guanidine hydrochloride

-
-
80267-19-2
5-(4-butoxy-3-methoxy-benzyl)-pyrimidine-2,4-diamine

| Conditions | Yield |
|---|---|
| With sodium methylate In ethanol for 24h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In 1,4-dioxane for 2h; Heating; | 98% |
| With hydrazine hydrate In 1,4-dioxane for 2h; Reflux; | 98% |
| With hydrazine hydrate In isopropyl alcohol for 6h; Reflux; | 97% |
-
-
50-01-1
guanidine hydrochloride

-
-
657408-23-6
5-bromo-4-nitro-1-(2'-deoxy-β-D-ribofuranosyl)imidazole

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrochloride With sodium methylate In methanol Stage #2: 5-bromo-4-nitro-1-(2'-deoxy-β-D-ribofuranosyl)imidazole In ethanol for 2h; Heating; | 98% |

-
-
50-01-1
guanidine hydrochloride

-
-
477845-12-8
4-(3-(4-chlorophenyl)benzo[c]isoxazol-5-yl)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With sodium In methanol at 20 - 80℃; for 18h; | 98% |
-
-
50-01-1
guanidine hydrochloride

-
-
7803-57-8
hydrazine hydrate

-
-
5329-29-3
triaminoguanidine hydrochloride

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Reflux; | 98% |
-
-
41792-58-9
1,1,3,6,8,8-Hexanitro-3,6-diaza-octan

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrochloride With sodium hydroxide In methanol at 20℃; for 0.166667h; Stage #2: 1,1,3,6,8,8-Hexanitro-3,6-diaza-octan In acetonitrile at 20℃; for 1h; | 98% |

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With caesium carbonate; dimethyl sulfoxide at 120℃; for 12h; Sealed tube; Schlenk technique; regioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 60℃; Sonication; Green chemistry; | 98% |

-
-
1679-39-6
3-chloro-2-methylpent-2-enal

-
-
50-01-1
guanidine hydrochloride

-
-
35733-54-1
4-ethyl-5-methylpyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol Reflux; | 98% |
-
-
78089-99-3
(E)-3-dimethylamino-1-(4-nitrophenyl)-2-propen-1-one

-
-
50-01-1
guanidine hydrochloride

-
-
99361-84-9
2-amino-4-(p-nitrophenyl)pyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrochloride With potassium carbonate In ethanol at 20℃; for 0.666667h; Stage #2: (E)-3-dimethylamino-1-(4-nitrophenyl)-2-propen-1-one In ethanol at 80℃; for 20h; | 97.3% |
| Stage #1: guanidine hydrochloride With sodium hydroxide In ethanol for 0.5h; Stage #2: (E)-3-dimethylamino-1-(4-nitrophenyl)-2-propen-1-one In ethanol at 40℃; for 12h; | 57% |

-
-
50-01-1
guanidine hydrochloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one at 120℃; | 97.2% |
-
-
50-01-1
guanidine hydrochloride

-
-
134373-03-8
tert-butyl 4-<5,5-dicyano-4-(dimethoxymethyl)pentyl>benzoate

-
-
134373-08-3
tert-butyl 4-<4-(2,4,6-triaminopyrimidin-5-yl)-5,5-dimethoxypentyl>benzoate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; tert-butyl alcohol for 2h; Heating; | 97% |
GUANIDINE HYDROCHLORIDE Chemical Properties
Product Name: Guanidine hydrochloride (50-01-1)
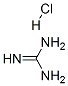
Molecular Formula: CH6ClN3
Molecular Weight: 95.53g/mol
Mol File: 50-01-1.mol
Einecs: 200-002-3
Melting Point: 180-185 °C(lit.)
Boiling point: 132.9 °C at 760 mmHg
Storage Temperature: Store at RT.
Flash Point: 34.2 °C
Density: 1.18 g/mL at 25 °C(lit.)
Refractive index: n20/D 1.465
Water Solubility: 2280 g/L (20 ºC)
Sensitive: Hygroscopic
Product Categories: Pharmaceutical Intermediates; Miscellaneous Biochemicals
Synonyms of Guanidine hydrochloride (50-01-1): guanidine,monohydrochloride ; guanidinechloride ; guanidinemonohydrochloride ; guanidiniumhydrochloride ; usafek-749 ; carbamidine hydrochloride ; iminourea hydrochloride ; guanidinium chloride .
GUANIDINE HYDROCHLORIDE Uses
Guanidine hydrochloride (50-01-1) is mainly used as a drug intermediate, is to create sulfadiazine, sulfa-methyl pyrimidine, sulfamethazine and folic acid an important raw material. It also can be used as medicine, pesticide, dyes and other organic synthesis intermediates. Can be used to synthesis of 2 - amino-pyrimidine, 2 - amino -6 - methyl-pyrimidine, 2 - amino -4,6 - dimethyl-pyrimidine, is to create sulfadiazine, sulfa-methyl pyrimidine, sulfamethazine and other sulfa drugs, intermediates. Guanidine (or guanidine nitrate) and ethyl cyanoacetate reaction, cyclization of 2,4 - 2-amino -6 - hydroxy-pyrimidine, folic acid used for synthesis of anti-anemia drugs. Also be used as synthetic anti-static agent.
GUANIDINE HYDROCHLORIDE Production
Double cyanide-ammonia and ammonium salt (ammonium chloride) as raw materials in the 170-230 ℃ melt under the reaction of guanidine hydrochloride were crude, after refining was finished.
GUANIDINE HYDROCHLORIDE Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | subcutaneous | 200mg/kg (200mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1352, 1935. | |
| guinea pig | LDLo | intraarterial | 500mg/kg (500mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 131, Pg. 171, 1928. | |
| guinea pig | LDLo | intravenous | 500mg/kg (500mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 131, Pg. 171, 1928. | |
| guinea pig | LDLo | subcutaneous | 100mg/kg (100mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1352, 1935. | |
| mammal (species unspecified) | LDLo | oral | 300mg/kg (300mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Industrial Hygiene. Vol. 13, Pg. 87, 1931. |
| mouse | LD50 | intraperitoneal | 500mg/kg (500mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | oral | 571mg/kg (571mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: IRRITABILITY | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 12, Pg. 598, 1993. |
| rabbit | LD | skin | > 2gm/kg (2000mg/kg) | Acute Toxicity Data. Journal of the American College of Toxicology, Part B. Vol. 12, Pg. 596, 1993. | |
| rat | LD50 | oral | 475mg/kg (475mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: EXCITEMENT GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | National Technical Information Service. Vol. AD-A165-747, |
| rat | LDLo | subcutaneous | 404mg/kg (404mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 28, Pg. 251, 1926. |
GUANIDINE HYDROCHLORIDE Safety Profile
Safety Information of Guanidine hydrochloride (50-01-1):
Hazard Codes: Xn,Xi![]()
Risk Statements: 22-36/38
22: Harmful if swallowed
36: Irritating to the eyes
38: Irritating to the skin
Safety Statements: 26-36-22
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
Related Products
- Guanidine acetate
- Guanidine carbonate
- Guanidine carbonate
- GUANIDINE HYDROCHLORIDE
- Guanidine monoperchlorate
- Guanidine nitrate
- Guanidine phosphate
- Guanidine sulfamate
- Guanidine, ((5,6-dihydro-3-hydroxy-1-methyl-6-oxo-5-indolinylidene)amino)-, monomethanesulfonate (salt)
- Guanidine, [2-(phosphonooxy)ethyl]- (9CI)
- 500112-74-3
- 500115-06-0
- 5001-18-3
- 500128-99-4
- 5001-33-2
- 5001-44-5
- 500148-38-9
- 5001-51-4
- 500160-56-5
- 500167-99-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View