-
Name
Gemfibrozil
- EINECS 247-280-2
- CAS No. 25812-30-0
- Article Data19
- CAS DataBase
- Density 1.044 g/cm3
- Solubility >0.5g/L(temperature not stated)
- Melting Point 61-63 °C
- Formula C15H22O3
- Boiling Point 394.7 °C at 760 mmHg
- Molecular Weight 250.338
- Flash Point 141.6 °C
- Transport Information
- Appearance White crystalline powder
- Safety 36-53-36/37-26-25
- Risk Codes 22-63-62-46-36/38-21
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeure;Lipira;Normolip;2,2-Dimethyl-5-(2,5-xylyloxy)valeric acid;Valeric acid, 2,2-dimethyl-5-(2,5-xylyloxy)-;WL-Gemfibrozil;Gozid;Litarek;Gemfibrozilum [INN-Latin];Pentanoic acid,5-(2,5-dimethylphenoxy)-2,2- dimethyl-;Apo-Gemfibrozil;Lipazil;Regulip;Lanaterom;Gemfibromax;Low-Lip;Gevilon Uno;Gemfibrozil [USAN:BAN:INN];Decrelip;Taborcil;Micolip;CI 719;Lifibron;Sinelip;Ipolipid;Reducel;2,2-Dimethyl-5-(2,5-xylyloxy)valeriansaeure;Gemfibrozil (JAN/USP);CI-719;Fibratol;Jezil;Brozil;Gemfibrozilo [INN-Spanish];Hipolixan;Gemd;Pilder;Gem-S;Lipur;5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acid;Fibrocit;Lopid;Pentanoic acid, 5-(2,5-dimethylphenoxy)-2,2-dimethyl-;Ausgem;Gemnpid;Clearol;
- PSA 46.53000
- LogP 3.57320
Synthetic route
-
-
169295-45-8
2,2-dimethyl-5-(2,5-dimethylphenoxy)-3-hydroxypentanoic acid β-lactone

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In N,N-dimethyl-formamide for 12h; Ambient temperature; | 90% |
-
-
139483-63-9
1,3-Bis[2.2-dimethyl-5-(2,5-dimethylphenoxy)-pentanoyloxy]-propane

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether; ethanol; water | 82% |

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; water; sodium hydroxide at 73 - 75℃; for 3h; Inert atmosphere; | 77.6% |
| With sodium hydroxide In dimethyl sulfoxide; toluene for 4h; | 860 mg |
-
-
39938-97-1
5-(2,5-dimethylphenoxy)-2,2-dimethylpentanal

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen In acetonitrile at 80℃; under 760.051 Torr; for 10h; Schlenk technique; | 72% |
| With potassium carbonate In n-heptane; water |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; sulfuric acid at -12 - -8℃; for 1.5h; | 56% |
-
-
201230-82-2
carbon monoxide

-
-
172533-96-9
2-methyl-5-(2,5-dimethylphenoxy)-2-pentene

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With copper(I) oxide; sulfuric acid at -12 - -8℃; | 47% |
-
-
201230-82-2
carbon monoxide

-
A
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With copper(I) oxide; sulfuric acid at 0 - 5℃; for 4h; | A n/a B 2.0 g |

-
-
201230-82-2
carbon monoxide

-
-
172533-96-9
2-methyl-5-(2,5-dimethylphenoxy)-2-pentene

-
A
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With sulfuric acid at -16℃; for 24h; | A 3.5 g B 1.5 g |


-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 66.9 percent / potassium bisulfite / 1.) from 50 to 60 deg C, 5 Torr, 5 min, 2.) from 60 to 120 deg C, 20 min 2: 47 percent / 1.) 98percent H2SO4, Cu2O / -12 - -8 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 66.9 percent / potassium bisulfite / 1.) from 50 to 60 deg C, 5 Torr, 5 min, 2.) from 60 to 120 deg C, 20 min 2: 3.5 g / 98percent H2SO4 / 24 h / -16 °C View Scheme |
-
-
95-87-4
2,5-Dimethylphenol

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 91 percent / aq. NaOH, tetrabutylammonium hydroxide / 24 h / Heating 2: 90 percent / aq. p-TsOH / ethyl acetate / 60 °C 3: 90 percent / ZnCl2 / ethyl acetate / 30 h / 4 °C 4: 90 percent / NaBH4 / dimethylformamide / 12 h / Ambient temperature View Scheme | |
| Multi-step reaction with 4 steps 1: 90 percent / aq. NaOH, tetrabutylammonium hydroxide / Heating 2: aq. p-TsOH / ethyl acetate / 0.75 h / 60 °C 3: 90 percent / ZnCl2 / ethyl acetate / 30 h / 4 °C 4: 90 percent / NaBH4 / dimethylformamide / 12 h / Ambient temperature View Scheme |
-
-
164917-45-7
3-(2,5-Dimethylphenoxy)-propionaldehyde

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / ZnCl2 / ethyl acetate / 30 h / 4 °C 2: 90 percent / NaBH4 / dimethylformamide / 12 h / Ambient temperature View Scheme |

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / aq. p-TsOH / ethyl acetate / 60 °C 2: 90 percent / ZnCl2 / ethyl acetate / 30 h / 4 °C 3: 90 percent / NaBH4 / dimethylformamide / 12 h / Ambient temperature View Scheme |
-
-
1025804-42-5
3-(2,5-dimethylphenoxy)propanal diethylacetal

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aq. p-TsOH / ethyl acetate / 0.75 h / 60 °C 2: 90 percent / ZnCl2 / ethyl acetate / 30 h / 4 °C 3: 90 percent / NaBH4 / dimethylformamide / 12 h / Ambient temperature View Scheme |
-
-
95-87-4
2,5-Dimethylphenol

-
-
139483-62-8
1,3-bis(2,2-dimethyl-5-chloropentanoyloxy)-propane

-
-
71-36-3
butan-1-ol

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With sodium hydroxide In hexane; water |

-
-
95-87-4
2,5-Dimethylphenol

-
-
139483-62-8
1,3-bis(2,2-dimethyl-5-chloropentanoyloxy)-propane

-
B
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium iodide In N-methyl-acetamide; water | |
| With sodium hydroxide; sodium iodide; dimethyl sulfoxide In hexane; water; toluene |


-
-
1313480-49-7
C23H29NO5

-
A
-
59-67-6
nicotinic acid

-
B
-
3612-80-4
2-Hydroxyethyl nicotinate

-
C
-
25812-30-0
gemfibrozil

-
D
-
1313404-96-4
2-hydroxyethyl 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoate

-
E
-
107-21-1
ethylene glycol

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 37℃; pH=7.4; Kinetics; aq. phosphate buffer; Enzymatic reaction; |

-
-
149105-26-0
isobutyl 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoate

-
A
-
25812-30-0
gemfibrozil

-
B
-
1446438-83-0
C30H42O6

-
C
-
1446438-84-1
C22H34O5

| Conditions | Yield |
|---|---|
| Stage #1: isobutyl 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoate With sodium hydroxide In toluene at 100 - 112℃; for 7h; Stage #2: With water at 70 - 80℃; | A 127.5 g B n/a C n/a |

-
-
16386-93-9
2,2-dimethylpent-4-enoic acid

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: dibenzoyl peroxide; hydrogen bromide / hexane / 5 h / 0 - 5 °C 2: sulfuric acid / 16 h / 60 - 70 °C 3: potassium carbonate; tetrabutylammomium bromide / toluene / 18 h / 110 °C / Inert atmosphere 4: tetrabutylammomium bromide; water; sodium hydroxide / 3 h / 73 - 75 °C / Inert atmosphere View Scheme |
-
-
82884-95-5
2,2-dimethyl-5-bromopentanoic acid

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid / 16 h / 60 - 70 °C 2: potassium carbonate; tetrabutylammomium bromide / toluene / 18 h / 110 °C / Inert atmosphere 3: tetrabutylammomium bromide; water; sodium hydroxide / 3 h / 73 - 75 °C / Inert atmosphere View Scheme |

-
A
-
25812-30-0
gemfibrozil

-
B
-
6554-98-9
trans-4-Hydroxystilbene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol |
-
-
698-27-1
4-methylsalicylaldehyde

-
-
82884-95-5
2,2-dimethyl-5-bromopentanoic acid

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 3h; Reflux; |
-
-
95-87-4
2,5-Dimethylphenol

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / toluene; dimethyl sulfoxide / 2 h / Reflux 1.2: 110 °C 2.1: sodium hydroxide / toluene; dimethyl sulfoxide / 4 h View Scheme |
-
-
73441-42-6
methyl 5-chloro-2,2-dimethylpentanoate

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / toluene; dimethyl sulfoxide / 2 h / Reflux 1.2: 110 °C 2.1: sodium hydroxide / toluene; dimethyl sulfoxide / 4 h View Scheme |
-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With 9-(2-chlorophenyl)acridine; chloropyridinecobaloxime(III) In dichloromethane; acetonitrile at 25 - 27℃; for 36h; Irradiation; regioselective reaction; | 99% |
-
-
524-38-9
N-hydroxyphthalimide

-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane | 98% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; Inert atmosphere; | 98% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 94% |
-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With iodine; oxygen; 9,10-phenanthrenequinone; trifluoroacetic acid In benzene at 25℃; for 1h; Irradiation; regioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine In acetonitrile for 3h; Reflux; | 98% |
-
-
25812-30-0
gemfibrozil

-
-
79791-29-0
2-(3-(2,5-dimethylphenoxy)propyl)-2-methylpropionyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 3h; | 97.5% |
| With thionyl chloride In dichloromethane at 40℃; for 1h; Inert atmosphere; | 72.9% |
| With oxalyl dichloride In dichloromethane at 0 - 20℃; Inert atmosphere; |
-
-
25812-30-0
gemfibrozil

-
-
39938-64-2
5-(2,5-dimethylphenoxy)-2,2-dimethylpentan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: gemfibrozil With borane-THF In tetrahydrofuran at 0 - 20℃; for 17h; Stage #2: With water In tetrahydrofuran | 97% |
| With borane-THF In tetrahydrofuran at 0 - 20℃; for 17h; | 97% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 70℃; for 12h; Cooling with ice; | 65.3% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 70℃; for 12h; | 37% |
-
-
25812-30-0
gemfibrozil

-
-
1119-51-3
bromopentene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; Inert atmosphere; | 97% |
-
-
25812-30-0
gemfibrozil

-
-
76456-06-9
imino(methyl)(pyridin-2-yl)-λ6-sulfanone

-
-
1384980-57-7
N-[5-(2,5-dmethylphenoxy)-2,2-dimethylpentanoyl]-S-methyl-S-2-pyridylsulfoximine

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 12h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: gemfibrozil With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane for 5.5h; Cooling with ice; Stage #2: 1-t-Butoxycarbonylpiperazine With triethylamine In dichloromethane for 12h; Cooling with ice; | 96% |
-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; caesium carbonate In N,N-dimethyl-formamide for 20h; Sealed tube; Irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 94% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 85% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 79% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 64.8% |
-
-
25812-30-0
gemfibrozil

-
-
1374011-92-3
5-(4-chloro-2,5-dimethylphenoxy)-2,2-dimethylvaleric acid

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; dimethyl sulfoxide In chloroform at 25℃; for 12h; Reagent/catalyst; Schlenk technique; | 93% |
| With sodium hypochlorite In methanol; water |
-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; N-acetyl-3-amino-2-methylpropanoic acid; sodium acetate; palladium diacetate In decane at 60℃; for 12h; | 93% |
| With tert.-butylhydroperoxide; N-acetyl-β-alanine; sodium acetate; palladium diacetate In water at 20 - 60℃; for 24.0833h; Sealed tube; |
-
-
25812-30-0
gemfibrozil

-
-
540-51-2
2-bromoethanol

-
-
1313404-96-4
2-hydroxyethyl 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Reflux; | 90.54% |
-
-
25812-30-0
gemfibrozil

-
-
65095-13-8
1-benzotriazolecarboxylic acid chloride

-
-
288576-82-9
gemfibrozil 1-benzotriazolide

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 20℃; for 2.5h; | 89.2% |
-
-
25812-30-0
gemfibrozil

-
-
536-38-9
4-chlorobenzoylmethyl bromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 20℃; for 12h; | 89.2% |
-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Stage #1: gemfibrozil With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 0 - 20℃; for 3h; Stage #2: With ammonia In dichloromethane at 0℃; for 0.5h; | 88.6% |
| Stage #1: gemfibrozil With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 3h; Stage #2: With ammonia In dichloromethane at 0℃; for 0.5h; | 88.6% |
-
-
25812-30-0
gemfibrozil

-
-
39938-97-1
5-(2,5-dimethylphenoxy)-2,2-dimethylpentanal

| Conditions | Yield |
|---|---|
| With sodium hydride; sodium iodide In tetrahydrofuran at 40℃; chemoselective reaction; | 88% |
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 17 h / 0 - 20 °C 2: pyridinium chlorochromate / dichloromethane / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: borane-THF / tetrahydrofuran / 17 h / 0 - 20 °C 2: pyridinium chlorochromate / dichloromethane / 16 h / 20 °C View Scheme | |
| Stage #1: gemfibrozil With 2,6-dimethylpyridine; (1,2-dimethoxyethane)dichloronickel(II); 4,4'-di-tert-butyl-2,2'-bipyridine; zinc; dimethyl dicarbonate In ethyl acetate for 0.25h; Inert atmosphere; Green chemistry; Stage #2: With diphenylsilane In ethyl acetate at 60℃; for 16h; Inert atmosphere; Green chemistry; | 196 mg |
-
-
25812-30-0
gemfibrozil

-
-
14737-80-5
N-methyl-tetrachlorophthalimide

| Conditions | Yield |
|---|---|
| With dmap; diisopropyl-carbodiimide In dichloromethane at 20℃; | 88% |
-
-
25812-30-0
gemfibrozil

-
-
1865-29-8
2-phenyl-acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With titanium(IV) dioxide In acetonitrile for 48h; Inert atmosphere; Irradiation; Cooling; | 87% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 2h; | 86% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 1h; Reflux; | 86% |
| With sulfuric acid Reflux; | 84% |
| With sulfuric acid at 80℃; for 16h; Inert atmosphere; | |
| With sulfuric acid |
-
-
25812-30-0
gemfibrozil

| Conditions | Yield |
|---|---|
| Stage #1: gemfibrozil With 2,6-dimethylpyridine; (1,2-dimethoxyethane)dichloronickel(II); 4,4'-di-tert-butyl-2,2'-bipyridine; zinc; dimethyl dicarbonate In ethyl acetate for 0.25h; Inert atmosphere; Green chemistry; Stage #2: With dideuteriodiphenylsilane In ethyl acetate at 60℃; for 16h; Inert atmosphere; Green chemistry; | 86% |

| Conditions | Yield |
|---|---|
| With (4s,6s)-2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile; sodium carbonate In dimethyl sulfoxide at 23 - 25℃; for 12h; Inert atmosphere; Schlenk technique; Sealed tube; Irradiation; | 86% |

-
-
25812-30-0
gemfibrozil

-
-
70-11-1
α-bromoacetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 20℃; for 12h; | 85.2% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 24h; Inert atmosphere; | 85% |
-
-
25812-30-0
gemfibrozil

-
-
2632-13-5
2-Bromo-4'-methoxyacetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 20℃; for 12h; | 84.2% |
-
-
25812-30-0
gemfibrozil

-
-
2495-35-4
benzylacrylate

| Conditions | Yield |
|---|---|
| With piperazine; 9-(2-chlorophenyl)acridine; tetrakis(acetonitrile)copper(I)tetrafluoroborate In dichloromethane at 25 - 27℃; for 14h; Irradiation; | 84% |
-
-
25812-30-0
gemfibrozil

-
-
619-41-0
4-(bromoacetyl)toluene

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 20℃; for 12h; | 83.7% |
Gemfibrozil Specification
1. Introduction of Gemfibrozil
Gemfibrozil is one kind of white or off-white crystalline powder. The IUPAC Name of this chemical is 5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic acid. It belongs to Active Pharmaceutical Ingredients;APIs;Bases & Related Reagents;Intermediates & Fine Chemicals;Nucleotides;Pharmaceuticals;Intracellular receptor.
The Classification Code of Gemfibrozil is Antihyperlipidemic; Antilipemic agents; Antimetabolites; Drug / Therapeutic Agent; Human Data; Hypolipidemic Agents; Lipid Regulating Agents; Reproductive Effect; Tumor data.
2. Properties of Gemfibrozil
Physical properties about Gemfibrozil are:
(1)Melting point: 61-63 °C; (2)Index of Refraction: 1.511; (3)Density: 1.044 g/cm3; (4)Flash Point: 141.6 °C; (5)Enthalpy of Vaporization: 68 kJ/mol; (6)Boiling Point: 394.7 °C at 760 mmHg; (7)Vapour Pressure: 6.13E-07 mmHg at 25 °C; (8)Appearance: White crystalline powder; (9)XLogP3-AA: 3.8; (10)H-Bond Donor: 1; (11)H-Bond Acceptor: 3.
3. Structure Descriptors of Gemfibrozil
(1)InChI: InChI=1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17)
(2)InChIKey: InChIKey=HEMJJKBWTPKOJG-UHFFFAOYSA-N
(3)Smiles: c1(c(ccc(c1)C)C)OCCCC(C(O)=O)(C)C
4. Toxicity of Gemfibrozil
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | oral | 2gm/kg (2000mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: OTHER CHANGES | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 25, Pg. 699, 1997. |
| man | TDLo | oral | 18gm/kg/3Y-I (18000mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | Archives of Internal Medicine. Vol. 151, Pg. 1873, 1991. |
| mouse | LD50 | oral | 2218mg/kg (2218mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Toksikologicheskii Vestnik. Vol. (6), Pg. 38, 1995. |
| rat | LD50 | intraperitoneal | 445mg/kg (445mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Toksikologicheskii Vestnik. Vol. (6), Pg. 38, 1995. |
| rat | LD50 | oral | 1414mg/kg (1414mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 25, Pg. 645, 1997. |
5. Physical Properties of Gemfibrozil
| Physical Property | Value | Units | Temp (deg C) | Source |
|---|---|---|---|---|
| Melting Point | 62 | deg C | EXP | |
| log P (octanol-water) | 4.770 | (none) | EST | |
| Atmospheric OH Rate Constant | 8.24E-11 | cm3/molecule-sec | 25 | EST |
Or
| 1. | orl-rat LD50:4786 mg/kg | PRSMA4 Proceedings of the Royal Society of Medicine. 69 (Suppl 2)(1976),15. | ||
| 2. | orl-mus LD50:3162 mg/kg | PRSMA4 Proceedings of the Royal Society of Medicine. 69 (Suppl 2)(1976),15. |
6. Safety information of Gemfibrozil
Hazard Codes:
 Xn,
Xn, Xi
Xi Risk Statements: 22-63-62-46-36/38-21
R21:Harmful in contact with skin.
R22:Harmful if swallowed.
R36/38:Irritating to eyes and skin.
R46:May cause heritable genetic damage.
R62:Risk of impaired fertility.
R63:Possible risk of harm to the unborn child.
Safety Statements: 36-53-36/37-26-25
S25:Avoid contact with eyes.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S36/37:Wear suitable protective clothing and gloves.
S53:Avoid exposure - obtain special instructions before use.
7. Uses of Gemfibrozil
Gemfibrozil (CAS NO.25812-30-0) is a serum lipid regulating agent used as an antihyperlipoproteinemic. It is a serum lipid regulating agent used as an antihyperlipoproteinemic. Gemfibrozil helps reduce cholesterol and triglycerides (fatty acids) in the blood. High levels of these types of fat in the blood are associated with an increased risk of atherosclerosis (clogged arteries).
8. Production of Gemfibrozil
(1)2,5-Dimethylphenol and 1-Bromo-3-chloropropane reaction of 1-(2,5-dimethylphenoxy)-3-chloropropane. The reaction is carried out in toluene, adding new clean off reflux 5h. Just as follows:
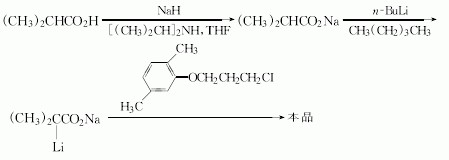
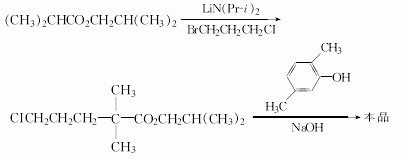
(2)N/A can be used to manufacture Gemfibrozil.
Related Products
- Gemfibrozil
- Gemfibrozil M3
- 25812-76-4
- 25813-25-6
- 2581-34-2
- 2581-43-3
- 25816-27-7
- 2581-69-3
- 25817-87-2
- 2582-30-1
- 25823-50-1
- 25823-53-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View