-
Name
Hydroxyurea
- EINECS 204-821-7
- CAS No. 127-07-1
- Article Data60
- CAS DataBase
- Density 1.457 g/cm3
- Solubility water: 50 mg/mL
- Melting Point 135-140 °C
- Formula CH4N2O2
- Boiling Point 136.04°C (rough estimate)
- Molecular Weight 76.055
- Flash Point
- Transport Information
- Appearance off-white crystalline solid
- Safety 53-36/37-45-36-22
- Risk Codes 46-63-61-40
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xn
Xn
- Synonyms Urea,hydroxy- (6CI,8CI,9CI);Biosupressin;Carbamohydroxamic acid;Carbamohydroximicacid;Carbamoyl oxime;Droxia;Hydrea;Hydreia;Hydroxycarbamide;Hydroxylamine, N-(aminocarbonyl)-;Hydura;Hydurea;Onco-Carbide;Oxyrea;Oxyurea;
- PSA 75.35000
- LogP 0.13510
Synthetic route
-
-
126669-78-1
N-(trimethylsiloxy)-N,N'-bis(trimethylsilyl)urea

-
-
127-07-1
N-hydroxyurea

| Conditions | Yield |
|---|---|
| With ethanol | 96% |
-
-
126669-76-9
N-(trimethylsiloxy)-N'-(trimethylsilyl)urea

-
-
127-07-1
N-hydroxyurea

| Conditions | Yield |
|---|---|
| With ethanol at 20℃; for 15h; | 94% |

| Conditions | Yield |
|---|---|
| With pyridine; hydrogenchloride; hydroxylamine hydrochloride; hydroxylamine In tetrahydrofuran; ethanol; dichloromethane; water; toluene | A n/a B 78% |


| Conditions | Yield |
|---|---|
| With hydroxylamine; sodium hydroxide In water at 20℃; for 3.7h; Time; | 62.3% |

| Conditions | Yield |
|---|---|
| With diethyl ether; hydroxylamine |

| Conditions | Yield |
|---|---|
| With hydroxylamine sulfate | |
| With hydroxylamine hydrochloride |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; water |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; water | |
| With Fe(3+), Cu(2+), H2SO4 In not given other Radiation; 20°C; vac.; X-ray irradiatin; concn. of urea 4.6 until 9.1 M in 0.1 N H2SO4; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydroxylamine hydrochloride | |
| With potassium hydroxide; hydroxylamine sulfate |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydroxylamine hydrochloride |
-
-
22509-74-6
N-ethoxycarbonylphthalimide

-
-
127-07-1
N-hydroxyurea

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium methylate |

| Conditions | Yield |
|---|---|
| With hydroxylamine In water at 30℃; Product distribution; Kinetics; different concentrations of NH2OH, reaction rate; also with Pseudomonas sp. CU6 cell extract (Km and Vmax) or without additives; |

| Conditions | Yield |
|---|---|
| With ethanol; hydroxylamine hydrochloride; triethylamine 1) temp. rising to 80 degC (exothermic), 2.) heating; Yield given. Multistep reaction; |

-
-
127-07-1
N-hydroxyurea

| Conditions | Yield |
|---|---|
| With ethanol; water Heating; |

| Conditions | Yield |
|---|---|
| at 25℃; Kinetics; Kinetik der Isomerisierung in gepufferten wss. Loesungen vom pH 9.2 bis pH 12.3; |

| Conditions | Yield |
|---|---|
| With ethanol at -15 - -10℃; Darstellung in mehreren Stufen; |

| Conditions | Yield |
|---|---|
| in gepufferter wss. Loesung vom pH 9.2 erfolgt Isomerisierung und Zersetzung; |


| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride |
-
-
590-28-3
potassium cyanate

-
-
7803-49-8
hydroxylamine

-
A
-
127-07-1
N-hydroxyurea

-
B
-
17479-46-8
1-Hydroxy-biuret

-
C
-
57-13-6
urea


| Conditions | Yield |
|---|---|
| With K cyanate byproducts: K2SO4; addn. of abs. alcohol carbamide formed; hydroxy carbamide formed on cooling the react mixt. and addn. a mixt. of alcohol and ether; filtered out K2SO4; | |
| With K cyanate byproducts: K2SO4; addn. of abs. alcohol carbamide formed; hydroxy carbamide formed on cooling the react mixt. and addn. a mixt. of alcohol and ether; filtered out K2SO4; |


| Conditions | Yield |
|---|---|
| In not given cooling; |
-
-
127-07-1
N-hydroxyurea

-
-
4996-21-8
1-(4-chloro-phenyl)-2,2-dihydroxy-ethanone

-
-
1118067-55-2
5-(4-chlorophenyl)-3-hydroxyimidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: N-hydroxyurea; 1-(4-chloro-phenyl)-2,2-dihydroxy-ethanone In acetonitrile at 20℃; for 51h; Stage #2: In acetonitrile Reflux; | 100% |
| With acetic acid at 18 - 19℃; for 24h; | 69% |

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; Inert atmosphere; | 100% |
-
-
127-07-1
N-hydroxyurea

-
-
14737-89-4
3,4-dimethoxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 2.5h; Inert atmosphere; | 100% |
-
-
127-07-1
N-hydroxyurea

-
-
16208-17-6
2,2-dihydroxy-1-(4-methoxyphenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With acetic acid at 19 - 20℃; for 96h; | 95% |
-
-
863718-32-5
4-chloro-2-(3,4,5-trimethoxy-phenyl)-5,6,7,8-tetrahydro-benzo[4,5]thieno[2,3-d]pyrimidine

-
-
127-07-1
N-hydroxyurea

-
-
1448530-58-2
1-hydroxy-3-[2-( 3 ,4 ,5-trimethoxyphenyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl]urea

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.0125h; Microwave irradiation; | 93% |
-
-
127-07-1
N-hydroxyurea

-
-
139491-37-5
methyl (5'S)-N-<1,3-butadienyl>-2'-oxo-pyrrolidine-5'-carboxylate


| Conditions | Yield |
|---|---|
| With tetrapropylammonium periodate; 4 A molecular sieve In methanol; dichloromethane at 0℃; for 16h; | A 28% B 92% |

-
-
127-07-1
N-hydroxyurea

-
-
76981-77-6
ethyl 2-cinnamamido-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate

-
-
1448530-47-9
(E)-N-hydroxy-4-oxo-2-styryl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-3(4H)-carboxamide

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; trichlorophosphate for 5h; Reflux; | 92% |
-
-
127-07-1
N-hydroxyurea

-
-
101130-32-9
4-chloro-2-styryl-5,6,7,8-tetrahydrobenzothieno<2,3-d>pyrimidine

-
-
1448530-59-3
(E)-1-hydroxy-3-(2-styryl-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4-yl)urea

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.0125h; Microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 6h; Reflux; | 91% |
| With potassium hydroxide In methanol Reflux; | 80% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 6h; Reflux; Inert atmosphere; | 91% |
| With potassium hydroxide In methanol for 6h; Reflux; | 91% |
| With potassium hydroxide In methanol for 6h; Reflux; | 91% |
| With potassium hydroxide In methanol for 6h; Reflux; | 91% |
-
-
127-07-1
N-hydroxyurea

-
-
1165952-91-9
cyclohexa-1,3-diene

| Conditions | Yield |
|---|---|
| With pyridine; copper(l) chloride In tetrahydrofuran at 20℃; for 2h; | 89% |
| With phosphate buffer; horseradish peroxidase at 20℃; for 1h; pH=7.4; | 42% |
| With Dess-Martin periodane In dichloromethane; N,N-dimethyl-formamide at 20℃; for 1.5h; Oxidation; cycloaddition; | 39% |
| With D-glucose; glucose oxidase; bovine liver catalase In phosphate buffer at 20℃; for 3h; pH=7.4; |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 18 - 20h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol Reflux; | 84% |
-
-
127-07-1
N-hydroxyurea

| Conditions | Yield |
|---|---|
| In ethanol for 16h; Reflux; | 84% |
-
-
127-07-1
N-hydroxyurea

-
-
158440-71-2
[14C]-Irofulven

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; acetone at 20℃; for 1h; | 83% |
| With sulfuric acid In acetone at 20℃; for 1h; Inert atmosphere; | 83% |
| With sulfuric acid In water; acetone at 20℃; for 24h; | 61% |
-
-
51868-95-2
1-(benzo[b]thiophen-2-yl)ethanol

-
-
127-07-1
N-hydroxyurea

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate; acetic acid In water at 40 - 45℃; for 6h; Product distribution / selectivity; | 82% |
| With hydrogenchloride In tetrahydrofuran; water at 50℃; for 4h; | 59.7% |
| With zinc(II) chloride at 135 - 140℃; for 1h; Product distribution / selectivity; | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol Reflux; | 82% |
-
-
127-07-1
N-hydroxyurea

-
-
115-37-7
thebaine

-
-
75848-83-8
6β,14β-(N-carbamoylepoxyimino)-6,14-dihydrothebaine

| Conditions | Yield |
|---|---|
| With sodium periodate; sodium acetate In water; ethyl acetate at 0℃; for 0.5h; | 79% |
-
-
127-07-1
N-hydroxyurea

-
-
638-10-8
ethyl 3-methylbut-2-enoate

-
-
62243-00-9
5,5-dimethyl-3-isoxazolidinone

| Conditions | Yield |
|---|---|
| With potassium methanolate In methanol at 20℃; for 2h; | 78.3% |
-
-
127-07-1
N-hydroxyurea

-
-
1074-12-0
phenylglyoxal hydrate

-
-
1034164-79-8
3-hydroxy-5-phenylimidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With water at 18℃; for 24h; | 77% |
-
-
72625-07-1
ethyl 2-(3,4,5-trimethoxybenzamido)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate

-
-
127-07-1
N-hydroxyurea

-
-
1448530-34-4
N-hydroxy-4-oxo-2-(3,4,5-trimethoxyphenyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine-3(4H)-carboxamide

| Conditions | Yield |
|---|---|
| With phosphorus pentoxide; trichlorophosphate for 5h; Reflux; | 77% |
-
-
127-07-1
N-hydroxyurea

-
-
80352-42-7
1-(4-bromophenyl)-2,2-dihydroxyethanone

| Conditions | Yield |
|---|---|
| With acetic acid at 17 - 18℃; for 26h; | 77% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol Reflux; | 76% |
-
-
127-07-1
N-hydroxyurea

-
-
1075-06-5
2,2-dihydroxy-1-phenyl-ethanone

-
-
1034164-79-8
3-hydroxy-5-phenylimidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With acetic acid at 18 - 19℃; for 24h; | 76% |
| Stage #1: N-hydroxyurea; 2,2-dihydroxy-1-phenyl-ethanone In water at 20℃; Stage #2: In water Heating; |
-
-
127-07-1
N-hydroxyurea

-
-
4998-15-6
4-chlorophenylglyoxal

-
-
1118067-55-2
5-(4-chlorophenyl)-3-hydroxyimidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With water | 74% |
-
-
127-07-1
N-hydroxyurea

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
A
-
77094-88-3
ureidoxymaleate de methyle

-
B
-
77094-89-4
ureidoxyfumarate de methyle

-
C
-
10068-07-2
methyl 3-hydroxy-5-isoxazolecarboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol for 0.333333h; Ambient temperature; | A 20% B 7% C 73% |
| With triethylamine In methanol for 0.333333h; Ambient temperature; | A 20% B 7% C 73% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol Reflux; | 73% |
Hydroxyurea Consensus Reports
Hydroxyurea Specification
The Hydroxyurea, with the CAS registry number 127-07-1, is also known as Urea, N-hydroxy-. It belongs to the product categories of Miscellaneous Biochemicals; Pharmacetical; Aliphatics; Nucleotides and Nucleosides; Hydroxylamines; Hydroxylamines (N-Substituted); Bases & Related Reagents; Nitric Oxide Reagents; Nucleotides; Carbonyl Compounds; Organic Building Blocks; Ureas; DNA Synthesis Inhibitors; DNA Synthesis Inhibitors Cancer Research; Antitumor Agents; Apoptosis and Cell Cycle; DNA Metabolism; Aids Aided Treatment; Amines. Its EINECS registry number is 204-821-7. This chemical's molecular formula is CH4N2O2 and molecular weight is 76.05. What's more, its IUPAC name is called 1-Hydroxyurea. It should be stored in a cool, dry and well-ventilated place. This chemical can be prepared by Ethyl Carbamate with hydroxylamine hydrochloride.
Physical properties about Hydroxyurea are: (1)ACD/LogP: -1.8; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.80; (4)ACD/LogD (pH 7.4): -1.80; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 2.50; (8)ACD/KOC (pH 7.4): 2.49; (9)#H bond acceptors: 4; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 75.35 Å2; (13)Index of Refraction: 1.501; (14)Molar Refractivity: 15.376 cm3; (15)Molar Volume: 52.161 cm3; (16)Polarizability: 6.096×10-24 cm3; (17)Surface Tension: 69.72 dyne/cm; (18)Density: 1.458 g/cm3.
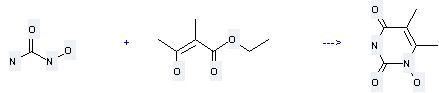
Uses of Hydroxyurea: (1) it is used as an anticancer drug; (2) it is used to produce other chemicals. For example, it can react with 2-methyl-3-oxo-butyric acid ethyl ester to get 1-hydroxy-5,6-dimethyl-1H-pyrimidine-2,4-dione. This reaction needs reagent NaOEt and solvent ethanol at ambient temperature. The reaction time is 72 hours. The yield is 60 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause damage to health at low levels and may cause heritable genetic damage. It has a carcinogenic effect and may cause harm to the unborn child. Therefore, you should wear suitable protective clothing and gloves. In addition, you should avoid exposuring and obtain special instructions before using it. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(N)NO
(2) InChI: InChI=1S/CH4N2O2/c2-1(4)3-5/h5H,(H3,2,3,4)
(3) InChIKey: VSNHCAURESNICA-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | > 1gm/kg (1000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| dog | LD50 | oral | > 2gm/kg (2000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| human | TDLo | intravenous | 86mg/kg (86mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BLOOD: OTHER CHANGES | Cancer Chemotherapy Reports, Part 1. Vol. 57, Pg. 369, 1973. |
| human | TDLo | oral | 80mg/kg/D (80mg/kg) | BLOOD: NORMOCYTIC ANEMIA BLOOD: LEUKOPENIA BLOOD: THROMBOCYTOPENIA | Cancer Chemotherapy Reports. Vol. 29, Pg. 103, 1963. |
| man | TDLo | oral | 14mg/kg (14mg/kg) | Annals of Pharmacotherpy. Vol. 29, Pg. 132, 1995. | |
| man | TDLo | oral | 450mg/kg/21D- (450mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING LUNGS, THORAX, OR RESPIRATION: "FIBROSIS, FOCAL (PNEUMOCONIOSIS)" | Netherlands Journal of Medicine. Vol. 51, Pg. 110, 1997. |
| man | TDLo | unreported | 64mg/kg/3D-I (64mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: COUGH | Australian and New Zealand Journal of Medicine. Vol. 28, Pg. 347, 1998. |
| mouse | LD10 | subcutaneous | 2400mg/kg (2400mg/kg) | European Journal of Cancer. Vol. 10, Pg. 667, 1974. | |
| mouse | LD50 | intraperitoneal | 5800mg/kg (5800mg/kg) | Cancer Research. Vol. 39, Pg. 3575, 1979. | |
| mouse | LD50 | intravenous | 2350mg/kg (2350mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| mouse | LD50 | oral | 7330mg/kg (7330mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| rat | LD50 | intraperitoneal | > 4700mg/kg (4700mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 18, Pg. 645, 1968. | |
| rat | LD50 | intravenous | 4730mg/kg (4730mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| rat | LD50 | oral | 5760mg/kg (5760mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 23, Pg. 682, 1992. | |
| rat | LD50 | unreported | 4300mg/kg (4300mg/kg) | Proceedings of the American Association for Cancer Research. Vol. 4, Pg. 10, 1963. | |
| women | TDLo | oral | 600mg/kg/30D- (600mg/kg) | Netherlands Journal of Medicine. Vol. 51, Pg. 110, 1997. | |
| women | TDLo | oral | 600mg/kg/30D- (600mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Netherlands Journal of Medicine. Vol. 51, Pg. 110, 1997. |
Related Products
- Hydroxyurea
- 127075-47-2
- 12707-58-3
- 127-08-2
- 127087-87-0
- 127-09-3
- 127095-92-5
- 127095-97-0
- 12709-64-7
- 1270-98-0
- 127104-68-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View