-
Name
L-Phenylglycinol
- EINECS 221-674-4
- CAS No. 3182-95-4
- Article Data210
- CAS DataBase
- Density 1.077 g/cm3
- Solubility
- Melting Point 92-94 °C(lit.)
- Formula C9H13NO
- Boiling Point 303.8 °C at 760 mmHg
- Molecular Weight 151.208
- Flash Point 137.5 °C
- Transport Information UN 3259 8/PG 3
- Appearance white to light yellow crystal powder
- Safety 26-36/37/39-45-24/25-27
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 1-Propanol, 2-amino-3-phenyl-, L-;(S)-beta-Aminobenzenepropanol;(S)-2-Benzylethanolamine;[(2S)-1-hydroxy-3-phenyl-propan-2-yl]azanium;2-amino-3-phenyl-propan-1-ol;L-2-Amino-3-phenylpropan-1-ol;L-2-Amino-3-phenyl-1-propanol;L-Phenylalainol;(R)/(S)-Phenylalaninol;L(-)-2-Amino-3-phenyl-1-propanol;L-(-)-Phenylalaninol;L- Phenylalaninol;(S)-(-)-2-amino-3-phenyl-1-propanol (L-phenylalaninol);L-(S)-Phenylalaninol;(S)-(-)-2-Amino-3-phenyl-1-propanol;(S)-2-amino-3-phenyl-1-propanol;H-Phe-ol;L(-)-Phenylalaninol;H-Phenylalaninol;Benzenepropanol, beta-amino-, (S)- (9CI);L-Phenylalaninol;S-Phenylalaninol;L-2-Phenylalaninol;
- PSA 46.25000
- LogP 1.24900
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran Heating; | 100% |
| With lithium borohydride; chloro-trimethyl-silane In tetrahydrofuran at 0 - 20℃; for 16h; | 99% |
| With sodium tetrahydroborate; iodine In tetrahydrofuran at 0℃; for 18.5h; Inert atmosphere; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; diborane In tetrahydrofuran at 20℃; for 1h; | 100% |
| With hydrogen In ethanol at 110℃; under 30003 Torr; Reagent/catalyst; Autoclave; chemoselective reaction; | 91.1% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 4h; Heating; | 86% |

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate at 23℃; for 2160h; Reduction; | 99% |

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol for 0.0833333h; | 98% |
| With hydrogen; palladium on activated charcoal In tetrahydrofuran; methanol under 760 Torr; for 3h; Ambient temperature; |
-
-
129397-83-7
(9-fluorenylmethoxycarbonyl)-L-phenylalaninol

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With piperidine In dichloromethane for 1h; Ambient temperature; | 85% |

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate at 23℃; for 120h; Reduction; | 85% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 18h; Reflux; | 85% |
-
-
207676-65-1
(-)-3-[2-(1-hydroxy-3-phenylpropyl)]-4-phenyloxazolidin-2-one

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With ammonia; lithium at -78℃; for 0.0833333h; | 81% |
-
-
90719-32-7
(S)-4-Benzyl-2-oxazolidinone

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 100℃; for 72h; | 80% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol 1.) room temperature, 4 h, 2.) reflux, 6 h, 3.) room temperature, overnight; | 78% |
| With sodium tetrahydroborate In ethanol; water for 18h; Heating; | 74% |
| Stage #1: methyl (2S)-2-amino-3-phenylpropanoate hydrochloride With triethylamine In methanol; diethyl ether at -10℃; for 1h; Stage #2: With sodium tetrahydroborate In methanol at 0 - 20℃; | 2.72 g |
| Stage #1: methyl (2S)-2-amino-3-phenylpropanoate hydrochloride With sodium tetrahydroborate In water at 0 - 5℃; for 16h; Stage #2: With hydrogenchloride In water pH=5; Stage #3: With sodium hydroxide In water for 3h; Reflux; | 50 g |
-
-
66605-57-0
(S)-N-tert-butoxycarbonyl-2-amino-3-phenylpropanol

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With sodium dodecyl-sulfate; benzyl bromide In water at 45 - 50℃; for 48h; chemoselective reaction; | 76% |
| With trifluoroacetic acid Substitution; | |
| In trifluoroacetic acid for 0.5h; | |
| With trifluoroacetic acid In dichloromethane |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol; water for 4.5h; Reflux; | 72% |
| With sodium tetrahydroborate In ethanol for 5.5h; Heating; | 71.7% |
| With sodium tetrahydroborate In ethanol | |
| With sodium tetrahydroborate In ethanol; water for 4h; Heating; Yield given; | |
| With sodium tetrahydroborate In ethanol; water for 4.5h; Reflux; | 19.16 g |
-
-
912480-01-4
(S)-dibenzyl 1-(1-hydroxy-3-phenylpropan-2-yl)-hydrazine-1,2-dicarboxylate

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With hydrogen; nickel; acetic acid In methanol at 20℃; under 3102.97 Torr; for 16h; | 70% |

| Conditions | Yield |
|---|---|
| With C24H20ClN2OPRu; potassium tert-butylate; hydrogen In tetrahydrofuran at 110℃; under 2280.15 - 30402 Torr; for 48h; Catalytic behavior; Solvent; Pressure; Temperature; Inert atmosphere; Schlenk technique; Autoclave; | 63% |
-
-
58745-46-3
(S)-N-(1-hydroxy-3-phenylpropan-2-yl)picolinamide

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-N-(1-hydroxy-3-phenylpropan-2-yl)picolinamide With hydrogenchloride In water at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: With zinc In water at 20℃; for 16h; Inert atmosphere; | 60% |
-
-
123366-90-5
(S)-3-phenyl-2-(1H-pyrrol-1-yl)propan-1-ol

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-3-phenyl-2-(1H-pyrrol-1-yl)propan-1-ol With ozone In methanol at -78℃; for 2.5h; Stage #2: With thiourea In methanol at 0 - 78℃; for 1.5h; Reagent/catalyst; Solvent; Inert atmosphere; | 60% |
-
-
17585-69-2, 27172-85-6, 28069-46-7, 74496-49-4, 79541-99-4
L-phenylalanine hydrochloride

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 12h; Reflux; Inert atmosphere; | 46.1% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; ammonium acetate; AmDHK78S/N276T In water at 40℃; for 24h; pH=9; Enzymatic reaction; | 41% |

| Conditions | Yield |
|---|---|
| Stage #1: L-phenylalanine With sodium tetrahydroborate; sulfuric acid In tetrahydrofuran; diethyl ether at 0 - 20℃; for 20h; Inert atmosphere; Stage #2: With hydrogenchloride In water at 20℃; for 2h; | A 26% B 36% |
-
-
3182-93-2
(L)-phenylalanine ethyl ester hydrochloride

-
A
-
3182-95-4
L-Phenylalaninol

-
B
-
5267-64-1
(R)-2-amino-3-phenylpropanol

| Conditions | Yield |
|---|---|
| With methanol; Na/SiO2 In tetrahydrofuran at 0 - 25℃; Bouveault-Blanc reduction; Inert atmosphere; optical yield given as %ee; | A 27% B n/a |
-
-
1795-98-8, 3182-95-4, 5267-64-1, 16088-07-6
rac-phenylalaninol

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| Stage #1: rac-phenylalaninol With O,O'-dibenzoyl-L-tartaric acid In acetone at 25℃; for 6h; Stage #2: With potassium hydroxide In dichloromethane | 24% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether | |
| With lithium aluminium tetrahydride In diethyl ether | |
| With lithium aluminium tetrahydride | |
| With sodium tetrahydroborate In ethanol; water |

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| (i) aq. NaBH4, (ii) (hydrolysis); Multistep reaction; |
-
-
91339-66-1
(S)-(-)-2-amino-1-hydroxy-3-phenylpropane ethyl ester hydrochloride

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol; water for 4h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| With α-chymotrypsin chemically modified with monomethoxypolyethylene glycol (α-CT-PEG) Rate constant; enzymatic hydrolysis at pH 8.0 (phosphate buffer), Km value; |
-
-
162334-16-9
L-phenylalaninol phenylacetate

-
A
-
103-82-2
phenylacetic acid

-
B
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With α-chymotrypsin chemically modified with monomethoxypolyethylene glycol (α-CT-PEG) Rate constant; enzymatic hydrolysis at pH 8.0 (phosphate buffer), Km value; |

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 85 - 90℃; for 48h; Yield given; |

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 85 - 90℃; for 48h; |
-
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

-
A
-
3182-95-4
L-Phenylalaninol

-
B
-
131288-67-0
(2S)-2-amino-3-cyclohexyl-1-propanol

| Conditions | Yield |
|---|---|
| With hydrogen; Nishimura catalyst <(45.9% Rh/19.9% Pt) oxide> In methanol at 25℃; under 75007.5 Torr; | A 40 % Spectr. B 40 % Spectr. |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
3182-95-4
L-Phenylalaninol

-
-
66605-57-0
(S)-N-tert-butoxycarbonyl-2-amino-3-phenylpropanol

| Conditions | Yield |
|---|---|
| In dichloromethane Acylation; | 100% |
| In ethanol at 20℃; | 100% |
| In water at 30 - 35℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol for 5h; Ambient temperature; | 100% |
-
-
3182-95-4
L-Phenylalaninol

-
-
1148-11-4
N-Benzyloxycarbonyl-L-proline

-
-
88084-14-4
benzyl 2-[(1-benzyl-2-hydroxyethyl)carbamoyl]pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran at 25℃; for 2.5h; | 100% |
| Stage #1: N-Benzyloxycarbonyl-L-proline With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran; N,N-dimethyl-formamide at -9 - -5℃; for 0.1h; Stage #2: L-Phenylalaninol In tetrahydrofuran; N,N-dimethyl-formamide at -5 - 20℃; for 20h; Solvent; Temperature; Reagent/catalyst; | 95% |
| With 4-methyl-morpholine; chloroformic acid ethyl ester In ethyl acetate at -15 - 20℃; for 15h; Inert atmosphere; Schlenk technique; | 75% |
| Stage #1: N-Benzyloxycarbonyl-L-proline With TEA; chloroformic acid ethyl ester In tetrahydrofuran at 0℃; for 0.5h; Stage #2: L-Phenylalaninol In tetrahydrofuran for 20h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethyl acetate at 120 - 130℃; for 3h; | 100% |
| With potassium carbonate In ethanol at 130 - 135℃; for 1h; | 88% |
| With potassium carbonate at 135 - 140℃; for 2h; | 84% |
-
-
85-44-9
phthalic anhydride

-
-
3182-95-4
L-Phenylalaninol

-
-
70451-01-3
(-)-(S)-2-(1-benzyl-2-hydroxyethyl)-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 145℃; for 41h; | 100% |
| With triethylamine In toluene for 3h; Condensation; Heating; | 96% |
| at 200℃; for 3h; | 94% |
-
-
3182-95-4
L-Phenylalaninol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
221045-94-9
(S)-(-)-2-amino-3-phenyl-1-(tert-butyldimethylsilyloxy)propane

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 21h; Substitution; | 100% |
| With triethylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; Cooling; | 89% |
| With dmap; triethylamine In dichloromethane at 20℃; | 88% |
-
-
3182-95-4
L-Phenylalaninol

-
-
99-63-8
benzene-1,3-dicarbonyl dichloride

-
-
475110-10-2
N1,N3-bis[(S)-1-hydroxy-3-phenylpropan-2-yl]isophthalamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 12h; | 100% |
| With triethylamine In dichloromethane at 0 - 25℃; for 8h; | 70% |
| With triethylamine In dichloromethane at 0 - 20℃; | 53% |
-
-
89-95-2
2-methyl-benzyl alcohol

-
-
3182-95-4
L-Phenylalaninol

-
-
850449-41-1
2-{[(E)-(S)-1-Hydroxymethyl-2-phenyl-ethylimino]-methyl}-phenol

| Conditions | Yield |
|---|---|
| With sodium sulfate In methanol for 12h; Heating; | 100% |
| In ethanol at 20℃; | |
| With magnesium sulfate In methanol Heating; | |
| In methanol |
-
-
3182-95-4
L-Phenylalaninol

-
-
839697-82-4
3-(triisopropylsilyl)propiolic acid

-
-
839698-01-0
(1S)-3-(triisopropylsilanyl)propynoic acid (1-benzyl-2-hydroxyethyl)amide

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 20h; | 100% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
3182-95-4
L-Phenylalaninol

-
-
176706-98-2
(4S,4S')-(-)-2,2'-(1-methylethylidene)bis[4,5-dihydro-4-(phenylmethyl)oxazole]

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In toluene for 48h; Heating; | 100% |
| With zinc(II) chloride In chlorobenzene for 24h; Inert atmosphere; Reflux; | 75% |
| With zinc(II) chloride In chlorobenzene for 72h; Reflux; | 24% |
-
-
3182-95-4
L-Phenylalaninol

-
-
63808-36-6
(2S)-2-cyanopyrrolidine-1-carboxylic acid benzyl ester

-
-
700875-99-6
(2S)-2-{(4S)-4-benzyl-4,5-dihydro-1,3-oxazol-2-yl}pyrrolidine-1-carboxylic acid benzyl ester

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In chlorobenzene Heating; | 100% |
-
-
3182-95-4
L-Phenylalaninol

-
-
50777-76-9
(2-formylphenyl)(diphenyl)phosphine

-
-
202345-92-4
(S)-2-(2-(diphenylphosphino)benzylideneamino)-3-phenylpropan-1-ol

| Conditions | Yield |
|---|---|
| With sodium sulfate In ethanol Heating; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 16h; | 100% |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
3182-95-4
L-Phenylalaninol

-
-
64889-55-0
(S)-2,2,2-trifluoro-N-(1-hydroxy-3-phenylpropan-2-yl)acetamide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 2.5h; | 100% |
-
-
34619-03-9
tert-butyldicarbonate

-
-
3182-95-4
L-Phenylalaninol

-
-
66605-57-0
(S)-N-tert-butoxycarbonyl-2-amino-3-phenylpropanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane; water at 20℃; for 24h; | 100% |
-
-
20632-57-9
3-[(phenylcarbamoyl)amino]benzoic acid

-
-
3182-95-4
L-Phenylalaninol

| Conditions | Yield |
|---|---|
| Stage #1: 3-[(phenylcarbamoyl)amino]benzoic acid With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: L-Phenylalaninol In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran at 20℃; Inert atmosphere; Cooling with ice; | 100% |

| Conditions | Yield |
|---|---|
| In toluene Reflux; Dean-Stark; stereoselective reaction; | 99.8% |
-
-
553-90-2
Dimethyl oxalate

-
-
3182-95-4
L-Phenylalaninol

-
-
19071-60-4, 133464-01-4
(S)-N,N'-bis<1-(hydroxymethyl)-2-phenylethyl>ethanediamide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.583333h; | 99% |
| In methanol at 20℃; for 0.5h; | 99% |
| In methanol at 20℃; for 0.0833333h; | 99% |
-
-
75-15-0
carbon disulfide

-
-
3182-95-4
L-Phenylalaninol

-
-
131744-19-9, 145588-94-9
(4S)-4-benzyl-1,3-oxazolidine-2-thione

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In ethanol at 50℃; | 99% |
| With 2-chloro-1,3-dimethylimidazolinium chloride; triethylamine In dichloromethane at 20℃; for 20h; Condensation; Cyclization; | 98% |
| With dihydrogen peroxide; potassium carbonate In ethanol at 50℃; for 0.0833333h; | 98% |
-
-
3182-95-4
L-Phenylalaninol

-
-
100431-28-5
1-chloro-2(S)-amino-phenylpropane hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide at 120℃; for 2h; | 99% |
| With hydrogenchloride; thionyl chloride |
-
-
3182-95-4
L-Phenylalaninol

-
-
79-22-1
methyl chloroformate

-
-
10289-05-1
N-[(1S)-2-Hydroxy-1-benzylethyl]methoxy carboxamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 20℃; Acylation; | 99% |
| With potassium carbonate In tetrahydrofuran; water at 0 - 20℃; for 2.16667h; Yield given; |
-
-
3182-95-4
L-Phenylalaninol

-
-
100-39-0
benzyl bromide

-
-
111060-52-7
(S)-2-(dibenzylamino)-3-phenylpropan-1-ol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 0℃; for 0.333333h; | 99% |
| With potassium carbonate In ethanol; water for 0.5h; Reflux; | 96% |
| Stage #1: L-Phenylalaninol With potassium carbonate In methanol; water for 0.166667h; Reflux; Stage #2: benzyl bromide In methanol; water for 1h; Heating; | 93% |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
3182-95-4
L-Phenylalaninol

-
-
216306-33-1
(S,2E)-2-((E)-3-phenylallylideneamino)-3-phenylpropan-1-ol

| Conditions | Yield |
|---|---|
| In diethyl ether for 1.33333h; Ambient temperature; | 99% |
| With magnesium sulfate In dichloromethane at 20℃; for 0.333333h; | 99% |
-
-
3182-95-4
L-Phenylalaninol

-
-
208051-70-1
2-methyl-4-methoxy-2H-1,2-benzothiazine-3-carboxylic acid 1,1-dioxide

-
-
208051-74-5
N-(2-methyl-4-methoxy-2H-1,2-benzothiazine-3-carbonyl)-L-phenylalaninol 1,1-dioxide

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; benzotriazol-1-ol | 99% |
-
-
5535-48-8
PVS

-
-
3182-95-4
L-Phenylalaninol

-
-
640296-86-2
(S)-3-phenyl-2-((2-(phenylsulfonyl)ethyl)amino)propan-1-ol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 48h; Heating; | 99% |
-
-
3182-95-4
L-Phenylalaninol

-
-
933-88-0
ortho-toluoyl chloride

-
-
497954-01-5
(2S)-2-o-toluamide-3-phenylpropanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dichloromethane at 0 - 20℃; | 99% |
| With triethylamine In tetrahydrofuran for 1h; |
L-Phenylglycinol Chemical Properties
Molecular structure of L-Phenylglycinol (CAS NO.3182-95-4) is:
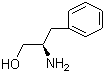
Product Name: L-Phenylglycinol
CAS Registry Number: 3182-95-4
IUPAC Name: 2-amino-3-phenylpropan-1-ol
Molecular Weight: 151.20562 [g/mol]
Molecular Formula: C9H13NO
XLogP3: 0.7
H-Bond Donor: 2
H-Bond Acceptor: 2
EINECS: 221-674-4
Melting Point: 92-94 °C(lit.)
Storage temp.: -15 °C
Sensitive: Air Sensitive
Surface Tension: 46.6 dyne/cm
Density: 1.077 g/cm3
Flash Point: 137.5 °C
Enthalpy of Vaporization: 57.46 kJ/mol
Boiling Point: 303.8 °C at 760 mmHg
Vapour Pressure: 0.0004 mmHg at 25°C
Product Categories: Pharmaceutical Intermediates;Amino Alcohols;chiral;Chiral Reagent;Phenylalanine [Phe, F];Amino Alcohols (Chiral);Chiral Building Blocks;for Resolution of Acids;Optical Resolution;Synthetic Organic Chemistry;Amino alcohols;Benzene derivatives
L-Phenylglycinol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | oral | > 1gm/kg (1000mg/kg) | Journal of Medicinal Chemistry. Vol. 11, Pg. 854, 1968. | |
| mouse | LD50 | intraperitoneal | 76mg/kg (76mg/kg) | Journal of Medicinal Chemistry. Vol. 20, Pg. 1578, 1977. |
L-Phenylglycinol Safety Profile
Safty information about L-Phenylglycinol (CAS NO.3182-95-4) is:
Hazard Codes:  C
C
Risk Statements: 34
R34:Causes burns.
Safety Statements: 26-36/37/39-45-24/25-27
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S24/25:Avoid contact with skin and eyes.
S27:Take off immediately all contaminated clothing.
RIDADR: UN 3259 8/PG 3
WGK Germany: 3
RTECS: UA6900000
F: 10-23
HS Code: 29221980
L-Phenylglycinol Specification
L-Phenylglycinol , its cas register number is 3182-95-4. It also can be called (S)-2-Benzylethanolamine ; (S)-beta-Aminobenzenepropanol ; L-2-Amino-3-phenyl-1-propanol ; S-Phenylalaninol ; 1-Propanol, 2-amino-3-phenyl-, L- ; Benzenepropanol, beta-amino-, (S)- (9CI) ; L-2-Amino-3-phenylpropan-1-ol .It is a white to light yellow crystal powder.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View