-
Name
Lithium acetate
- EINECS 208-914-3
- CAS No. 546-89-4
- Article Data19
- CAS DataBase
- Density 1.3[at 20℃]
- Solubility H2O: 5 M at 20 °C, clear, colorless
- Melting Point 280-285 °C
- Formula C2H3LiO2
- Boiling Point 117.1 °C at 760 mmHg
- Molecular Weight 65.9856
- Flash Point 40 °C
- Transport Information
- Appearance solid
- Safety 23-24/25-39-26-22
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Aceticacid, lithium salt (8CI,9CI);Lithium acetate (6CI);Lithium ethanoate;Quilone;Quilonorm;Quilonum;
- PSA 40.13000
- LogP -1.24380
Synthetic route
-
-
36649-96-4
lithium pentafluorobenzenesulfinate

-
-
1600-27-7
mercury(II) diacetate

-
A
-
17314-19-1
C6F5HgO(O)CCH3

-
B
-
546-89-4
lithium acetate

| Conditions | Yield |
|---|---|
| In water in aq. suspension at 25°C, molar ratio of LiOS(O)C6F5 and Hg comp. = 1.0:2.1, immediately react.; | A 55% B n/a |
| In water in aq. suspension at 25°C, molar ratio of LiOS(O)C6F5 and Hg comp. = 1.0:2.1, immediately react.; | A 55% B n/a |

-
-
14680-31-0
lithium tert-butyl hydroperoxide

-
-
67-64-1
acetone

-
A
-
50-00-0
formaldehyd

-
B
-
556-63-8
lithium formate

-
C
-
546-89-4
lithium acetate

-
D
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| lithium tert-butoxide In n-heptane at 40℃; Rate constant; Kinetics; other solvents and inhibitors investigated; Energy of activation, A-factor;; |

-
-
14680-31-0
lithium tert-butyl hydroperoxide

-
A
-
556-63-8
lithium formate

-
B
-
546-89-4
lithium acetate

-
C
-
1907-33-1
lithium tert-butoxide

-
D
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| In various solvent(s) at 80℃; for 8h; Yield given. Yields of byproduct given; | |
| In toluene; cyclohexene at 85℃; for 5h; Kinetics; Product distribution; other solvent, other temperature, other time; | |
| In toluene; cyclohexene at 85℃; for 5h; Yield given. Yields of byproduct given; |

-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
3240-34-4
[bis(acetoxy)iodo]benzene

-
A
-
542-69-8
1-iodo-butane

-
B
-
591-50-4
iodobenzene

-
C
-
111-65-9
octane

-
D
-
591-78-6
n-hexan-2-one

-
E
-
546-89-4
lithium acetate

-
F
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -5℃; for 0.0833333h; Product distribution; Mechanism; various amounts of nBuLi, other iodine compounds, various organolithium reagents; |


-
A
-
556-63-8
lithium formate

-
B
-
546-89-4
lithium acetate

-
C
-
1907-33-1
lithium tert-butoxide

-
D
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| 1-amino-naphthalene In n-heptane at 80℃; Thermodynamic data; Rate constant; other temperature, other solvent, reaction energy, activation entropy, activation enthalpy, gibbs function; |


| Conditions | Yield |
|---|---|
| In acetic acid equilibrium in glacial acetic acid;; |

-
-
6108-17-4, 61013-21-6, 61013-23-8, 67299-15-4, 116277-84-0
lithium acetate dihydrate

-
-
546-89-4
lithium acetate

| Conditions | Yield |
|---|---|
| In solid heating at 50-195°C; | |
| In neat (no solvent, solid phase) 110-180°C; XRD; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) Ar; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) thermolysis in the absence of O2; |

| Conditions | Yield |
|---|---|
| With perchloric acid; water; lithium perchlorate; 2-amino-2-hydroxymethyl-1,3-propanediol pH=7.3 - 8.9; Activation energy; Kinetics; Mechanism; Temperature; |

| Conditions | Yield |
|---|---|
| With lithium hexafluorophosphate; oxygen Electrochemical reaction; |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 125℃; for 120h; Inert atmosphere; | 5.2 g |

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide In water at 55℃; for 6h; Temperature; | 94.15% |

-
-
38689-22-4
hudrochloranilic acid

-
-
71-91-0
tetraethylammonium bromide

-
-
546-89-4
lithium acetate

-
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| In water | 94% |


| Conditions | Yield |
|---|---|
| With bis[2-(2-methoxyethoxy)ethyl] 4-oxo-4H-pyran-2,6-dicarboxylate In acetonitrile at 80℃; for 24h; Reagent/catalyst; | 94% |
-
-
66788-01-0
9,10-Dihydrobenzopyrene

-
-
546-89-4
lithium acetate

-
-
120624-77-3, 120708-42-1
(+/-)-trans-9-acetoxy-10-bromo-9,10,11,12-tetrahydrobenzopyrene

| Conditions | Yield |
|---|---|
| With N-bromoacetamide In acetic acid for 1.5h; Ambient temperature; | 92% |

-
-
546-89-4
lithium acetate

-
-
95012-61-6
tetra-μ-acetato-diruthenium(II,II)-bis(tetrahydrofuran)

| Conditions | Yield |
|---|---|
| In methanol Ru-salt was heated under reflux for 20 min in MeOH contg. Li(O2CMe) under Ar; the soln. was cooled to ambient temp., filtered off, recrystd. from THF at -20°C; | 92% |
-
-
546-89-4
lithium acetate

-
-
1165952-91-9
cyclohexa-1,3-diene

-
-
82736-39-8
cis-1-acetoxy-4-chlorocyclohex-2-ene

| Conditions | Yield |
|---|---|
| With p-benzoquinone; lithium chloride; palladium diacetate In water; acetic acid Ambient temperature; | 89% |
| With palladium diacetate; p-benzoquinone; lithium chloride In acetic acid at 20℃; for 5h; diastereoselective reaction; | 18.7 g |
-
-
546-89-4
lithium acetate

-
-
59040-31-2
1,4-but-2-ynoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With acetic acid-d; palladium diacetate for 14h; Ambient temperature; | 87% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
546-89-4
lithium acetate

-
-
67722-66-1
1,1'-(6,7,9,10,17,18,20,21-octahydrodibenzo[b,k]-[1,4,7,10,13,16]hexaoxacyclooctadecine-2,13-diyl)diethanone

| Conditions | Yield |
|---|---|
| With PPA at 68 - 72℃; for 2h; | 86% |
-
-
16880-79-8
1,2-Dihydroacridine

-
-
546-89-4
lithium acetate

| Conditions | Yield |
|---|---|
| With N-bromoacetamide In acetic acid for 4h; Ambient temperature; | 85% |
-
-
546-89-4
lithium acetate

-
-
151623-58-4
5,6-dihydro-naphthalene-2-carboxylic acid

-
-
1026061-78-8
8-Acetyl-7-bromo-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With N-bromoacetamide In acetic acid for 3h; Ambient temperature; | 85% |
-
-
546-89-4
lithium acetate

-
-
158604-47-8
O6-<2-(4-nitrophenyl)ethyl>-9-<3',5'-O-(1,1,3,3-tetraisopropyldisiloxane-1,3-diyl)-2'-O-<(trifluoromethyl)sulfonyl>-β-D-ribofuranosyl>guanine

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide In N,N-dimethyl-formamide Ambient temperature; | 85% |
-
-
546-89-4
lithium acetate

-
-
1165952-91-9
cyclohexa-1,3-diene

-
-
78776-44-0, 20117-77-5, 78776-45-1, 135615-69-9
trans-(±)-4-(acetoxy)cyclohex-2-en-1-yl acetate

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; iron(II) phthalocyanine; 2-(phenylsulfinyl)-1,4-benzoquinone In acetic acid under 760 Torr; for 5.5h; Mechanism; Ambient temperature; or 1,4-hydroquinone/DMSO , other benzoquinones, time course of the reaction, stereoselectivity, other diene; | 85% |
| With oxygen; palladium diacetate; iron(II) phthalocyanine; 2-(phenylsulfinyl)-1,4-benzoquinone In acetic acid under 760 Torr; for 5.5h; Ambient temperature; | 85% |
| With manganese(IV) oxide; palladium diacetate; acetic acid; p-benzoquinone In pentane at 25℃; for 72h; | 79% |

| Conditions | Yield |
|---|---|
| With bis[2-(2-methoxyethoxy)ethyl] 4-oxo-4H-pyran-2,6-dicarboxylate In acetonitrile at 80℃; for 24h; Reagent/catalyst; | 85% |
-
-
1121-63-7
cyclohepta-3,5-dien-1-ol

-
-
546-89-4
lithium acetate

-
-
134780-02-2
meso-(1R,4R,6R)-3,6-Diacetoxy-4-cyclohepten-1-ol

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; palladium diacetate; p-benzoquinone In acetic acid at 25℃; for 64h; | 84% |
| With manganese(IV) oxide; p-benzoquinone; palladium diacetate In acetic acid for 41h; Ambient temperature; | 75% |

| Conditions | Yield |
|---|---|
| palladium diacetate In acetic acid for 20h; Ambient temperature; | 83% |
| palladium diacetate In acetic acid Product distribution; or sodium, various times; other 2-alkynoic acid derivatives; |
-
-
546-89-4
lithium acetate

-
-
151623-57-3
7,8-Dihydronaphthalene-2-carboxylic acid

-
-
1026640-91-4
5-Acetyl-6-bromo-5,6,7,8-tetrahydronaphthalene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| With N-bromoacetamide In acetic acid for 3h; Ambient temperature; | 83% |
-
-
546-89-4
lithium acetate

-
-
4891-38-7
phenylpropynoic acid methyl ester

-
-
145733-73-9
(Z)-3-Acetoxy-3-phenyl-acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| palladium diacetate In acetic acid for 22h; Ambient temperature; | 83% |
-
-
546-89-4
lithium acetate

-
-
23326-27-4
Methyl 2-butynoate

-
-
4525-27-3
β-acetoxy-crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| palladium diacetate In acetic acid for 15h; Ambient temperature; | 83% |
-
-
546-89-4
lithium acetate

-
-
125974-32-5
1-Benzyl-3-(2-cyclohexa-2,4-dienyl-ethyl)-urea

-
-
125974-36-9, 125974-37-0
Acetic acid (3aS,7aS)-1-(toluene-4-sulfonyl)-2,3,3a,4,5,7a-hexahydro-1H-indol-5-yl ester

| Conditions | Yield |
|---|---|
| With palladium diacetate; p-benzoquinone In acetic acid; acetone for 16h; Ambient temperature; | 82% |
-
-
546-89-4
lithium acetate

-
-
37624-10-5
3,4-Dihydroacridine

| Conditions | Yield |
|---|---|
| With N-bromoacetamide In acetic acid for 4h; Ambient temperature; | 81% |
-
-
546-89-4
lithium acetate

-
-
603-36-1
triphenylantimony

-
-
1538-62-1, 34716-94-4
triphenylantimony(V) diacetate

| Conditions | Yield |
|---|---|
| In acetonitrile Electrolysis; N2 atmosphere, ambient temp.; constant current electrolysis (Pt plate and wire electrodes); extraction (ether), washing of extracts (water, brine), drying (MgSO4),solvent removal, recrystn. (ethanol); | 81% |

| Conditions | Yield |
|---|---|
| With N2H4 In water to Pu-compd. in H2O added with stirring CH3COOLi, N2H4 and Sr(NO3)2 in H2O, coagulated for ca. 30 min; filtered in vac., washed with cold H2O, dried in an air flow for 3 h, elem. anal.; | 80% |

-
-
546-89-4
lithium acetate

-
-
1165952-91-9
cyclohexa-1,3-diene

-
-
78776-45-1
cis-2-cyclohexenyl-1,4-diacetate

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; palladium diacetate; acetic acid; p-benzoquinone; lithium chloride In pentane at 25℃; for 72h; | 79% |
| With palladium diacetate; acetic acid In pentane |
-
-
546-89-4
lithium acetate

-
-
59040-31-2
1,4-but-2-ynoic acid benzyl ester

-
-
145733-71-7
(Z)-3-Acetoxy-but-2-enoic acid benzyl ester

| Conditions | Yield |
|---|---|
| palladium diacetate In acetic acid for 8.5h; Ambient temperature; | 78% |

-
-
546-89-4
lithium acetate

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 40℃; for 18h; Inert atmosphere; Sealed tube; | 78% |
-
-
546-89-4
lithium acetate

-
-
115522-58-2
6-(benzyloxy)-1,3-cycloheptadiene

-
-
115522-57-1, 150338-86-6
1β-acetoxy-4β-chloro-6α-(benzyloxy)-2-cycloheptene

| Conditions | Yield |
|---|---|
| With p-benzoquinone; lithium chloride; palladium diacetate In acetic acid | 77% |

| Conditions | Yield |
|---|---|
| With (1,4,7,10-tetraoxacyclododecane) In dimethyl sulfoxide for 10h; Reagent/catalyst; Reflux; | 77% |

| Conditions | Yield |
|---|---|
| palladium diacetate In trifluoroacetic acid for 5h; Ambient temperature; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: lithium acetate With bromobenzene; lithium; 1,1,1,3,3,3-hexamethyl-disilazane In tetrahydrofuran at 20℃; Stage #2: 2-Adamantanone In tetrahydrofuran at 20℃; for 2h; Reflux; Stage #3: With sulfuric acid In tetrahydrofuran; water Cooling; | 76% |
Lithium acetate Consensus Reports
Lithium acetate Standards and Recommendations
Lithium acetate Specification
The Lithium acetate, with the CAS registry number 546-89-4, is also known as Acetic acid, lithium salt (1:1). It belongs to the product categories of Organic-Metal Salt; Lithium Compounds; Classes of Metal Compounds; Li (Lithium) Compounds; Typical Metal Compounds; LithiumMicro/Nanoelectronics; Inorganic Salts; Lithium; Solution Deposition Precursors; Synthetic Reagents. Its EINECS registry number is 208-914-3. This chemical's molecular formula is C2H3LiO2 and molecular weight is 65.99. Its IUPAC name is called lithium acetate. Lithium acetate is used in the laboratory as buffer for gel electrophoresis of DNA and RNA.
Physical properties of Lithium acetate: (1)ACD/LogP: -0.29; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.07; (4)ACD/LogD (pH 7.4): -2.86; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 2.73; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)Flash Point: 40 °C; (12)Enthalpy of Vaporization: 23.7 kJ/mol; (13)Boiling Point: 117.1 °C at 760 mmHg; (14)Vapour Pressure: 13.9 mmHg at 25°C.
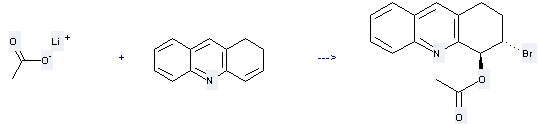
Uses of Lithium acetate: it can be used to produce trans-4-Acetoxy-3-bromo-1,2,3,4-tetrahydroacridine. This reaction will need reagent N-bromoacetamide and solvent acetic acid. The reaction time is 4 hours at ambient temperature. The yield is about 85%.
Uses of Lithium acetate: Lithium acetate is used in the laboratory as buffer for gel electrophoresis of DNA and RNA. It has a lower electrical conductivity and can be run at higher speeds than can gels made from TAE buffer (5-30V/cm as compared to 5-10V/cm). Lithium boric acid or sodium boric acid are usually preferable to lithium acetate or TAE when analyzing smaller fragments of DNA (less than 500 bp) due to the higher resolution of borate-based buffers in this size range as compared to acetate buffers. Lithium acetate is also used to permeabilize the cell wall of yeast for use in DNA transformation.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes. You should not breathe its gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer). What's more, you must avoid contact with skin and eyes. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Wear eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: [Li+].CC(=O)[O-]
(2)InChI: InChI=1S/C2H4O2.Li/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
(3)InChIKey: XIXADJRWDQXREU-UHFFFAOYSA-M
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | oral | 1500mg/kg (1500mg/kg) | Pharmacology and Toxicology. English translation of FATOAO. Vol. 21, Pg. 419, 1958. | |
| mouse | LDLo | subcutaneous | 1500mg/kg (1500mg/kg) | Pharmacology and Toxicology. English translation of FATOAO. Vol. 21, Pg. 419, 1958. |
Related Products
- Lithium acetate
- Lithium acetate dihydrate
- Lithium acetoacetate
- Lithium acetylacetonate
- Lithium acetylide
- Lithium acetylide ethylenediamine complex
- Lithium aluminate
- Lithium aluminium hydride
- Lithium amide
- Lithium antimony thiomalate
- 5468-96-2
- 5469-16-9
- 5469-26-1
- 546-93-0
- 5469-33-0
- 5469-36-3
- 5469-45-4
- 5469-47-6
- 5469-60-3
- 54696-05-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View