-
Name
Losartan potassium
- EINECS 200-287-4
- CAS No. 124750-99-8
- Article Data40
- CAS DataBase
- Density 0.986g/mLat 25°C(lit.)
- Solubility
- Melting Point ?69°C(lit.)
- Formula C22H22ClKN6OK
- Boiling Point 682 °C at 760mmHg
- Molecular Weight 461.008
- Flash Point 366.3 °C
- Transport Information
- Appearance white to off-white crystalline powder
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Lotim;L-158086;Ocsaar;Losacor;Tenopres;Lortaan;Losaprex;Hyzaar;Niten;DuP 753;Losartan monopotassium salt;MK 954;Lorzaan;Cozaar;Llosartan;Losartanpotassium;Lorzaar;1H-Imidazole-5-methanol,2-butyl-4-chloro- 1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]- methyl]-,monopotassium salt;2-Butyl-4-chloro-1-(2-(tetrazol-5-yl)biphenyl-4-ylmethyl)-1H-imidazole-5-methanol potassium;MK-0954;Losacar;MK 0954;potassium [2-butyl-5-chloro-3-[[4-[2-(2,3,4-triaza-1-azanidacyclopenta-2,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol;Cozaar (TN);
- PSA 89.61000
- LogP 3.89590
Synthetic route
-
-
114798-26-4
lorsartan

-
-
124750-99-8
losartan potassium

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; isopropyl alcohol at 20℃; for 0.5h; | 90% |
| With potassium hydroxide In methanol for 4h; Heating / reflux; | 33% |
| With potassium hydroxide In methanol for 1h; pH=9; Reflux; |
-
-
124750-67-0
2-n-Butyl-4-chloro-1-[(2'-cyanobiphenyl-4-yl)methyl]imidazole-5-carboxaldehyde

-
-
124750-99-8
losartan potassium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium tetrahydroborate / methanol; N,N-dimethyl acetamide / 1 h / 5 - 20 °C 2.1: sodium azide; triethylamine hydrochloride / N,N-dimethyl acetamide / 16 h / 120 °C 2.2: 80 °C / pH 4.0 3.1: potassium hydroxide / methanol; isopropyl alcohol / 0.5 h / 20 °C View Scheme |
-
-
83857-96-9
2-n-butyl-4-chloro-1H-imidazol-5-carboxaldehyde

-
-
124750-99-8
losartan potassium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl acetamide / 5 h / 20 °C 2.1: sodium tetrahydroborate / methanol; N,N-dimethyl acetamide / 1 h / 5 - 20 °C 3.1: sodium azide; triethylamine hydrochloride / N,N-dimethyl acetamide / 16 h / 120 °C 3.2: 80 °C / pH 4.0 4.1: potassium hydroxide / methanol; isopropyl alcohol / 0.5 h / 20 °C View Scheme |
-
-
114772-54-2
4'-(bromomethyl)-1,1'-biphenyl-2-carbonitrile

-
-
124750-99-8
losartan potassium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl acetamide / 5 h / 20 °C 2.1: sodium tetrahydroborate / methanol; N,N-dimethyl acetamide / 1 h / 5 - 20 °C 3.1: sodium azide; triethylamine hydrochloride / N,N-dimethyl acetamide / 16 h / 120 °C 3.2: 80 °C / pH 4.0 4.1: potassium hydroxide / methanol; isopropyl alcohol / 0.5 h / 20 °C View Scheme |
-
-
114772-55-3
2-n-Butyl-4-chloro-1-[(2'-cyanobiphenyl-4-yl)-methyl]-5-(hydroxymethyl)imidazole

-
-
124750-99-8
losartan potassium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium azide; triethylamine hydrochloride / N,N-dimethyl acetamide / 16 h / 120 °C 1.2: 80 °C / pH 4.0 2.1: potassium hydroxide / methanol; isopropyl alcohol / 0.5 h / 20 °C View Scheme |
-
-
124750-99-8
losartan potassium

-
-
124750-92-1
2-butyl-4-chloro-1-((2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: losartan potassium With pyridine; potassium permanganate; tetrabutyl-ammonium chloride In water; acetone at 40 - 50℃; for 1h; Stage #2: With hydrogenchloride In water; acetone Product distribution / selectivity; | 72% |
| Stage #1: losartan potassium With potassium hydroxide In water at 2℃; for 2h; Stage #2: With sodium periodate; ruthenium trichloride In water at 4 - 6.5℃; for 19h; |
-
-
76-83-5
trityl chloride

-
-
124750-99-8
losartan potassium

-
-
124751-00-4
2-Butyl-4-chloro-1-[[2'-[1-(triphenylmethyl)-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; water |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 60℃; for 72h; |
-
-
124750-99-8
losartan potassium

-
-
913611-27-5
2-butyl-4-chloro-1-[(2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl]-imidazole-5-carboxylic acid monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 2 h / 2 °C 1.2: 19 h / 4 - 6.5 °C 2.1: hydrogenchloride / acetic acid 3.1: water / 72 h / centrifuge tube View Scheme |
Losartan potassium Specification
The Losartan potassium is one kind of white to off-white crystalline powder. The IUPAC Name of this chemical is Potassium [2-butyl-5-chloro-3-[[4-[2-(2,3,4-triaza-5-azanidacyclopenta-1,3-dien-1-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol. Besides, Losartan potassium belongs to Hypertension; Intermediates & Fine Chemicals; Pharmaceuticals; Losartan. In addition, its Classification Code is Antihypertensive; Drug / Therapeutic Agent; Human Data; Reproductive Effect. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone. Losartan potassium can be used as a nonpeptide angiotensin II AT1-receptor antagonist. It is an antagonist of ANGIOTENSIN TYPE 1 RECEPTOR with antihypertensive activity due to the reduced pressor effect of ANGIOTENSIN II.The Losartan potassium is irritating to eyes, respiratory system and skin.When you use it ,wear suitable protective clothing, gloves and eye/face protection.In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Physical properties about Losartan potassium are:
(1)Flash Point: 366.3 °C; (2)Enthalpy of Vaporization: 105.1 kJ/mol; (3)Boiling Point: 682 °C at 760 mmHg; (4)Vapour Pressure: 1.55E-19 mmHg at 25 °C; (5)H-Bond Donor: 1; (6)H-Bond Acceptor: 6; (7)Rotatable Bond Count: 8; (8)Tautomer Count: 2; (9)Exact Mass: 460.118069; (10)MonoIsotopic Mass: 460.118069; (11)Topological Polar Surface Area: 77.7; (12)Heavy Atom Count: 31.
Structure Descriptors of Losartan potassium:
(1)Canonical SMILES: CCCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NN=N[N-]4)CO)Cl.[K+]
(2)InChI: InChI=1S/C22H22ClN6O.K/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22;/h4-7,9-12,30H,2-3,8,13-14H2,1H3;/q-1;+1
(3)InChIKey: OXCMYAYHXIHQOA-UHFFFAOYSA-N
(4)Smiles: n1(c(nc(c1CO)Cl)CCCC)Cc1ccc(c2c(c3nnn[n-]3)cccc2)cc1.[K+]
Toxicity of Losartan potassium as follows:
| dog | LD | oral | > 320mg/kg (320mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: NAUSEA OR VOMITING | Kiso to Rinsho. Clinical Report. Vol. 28, Pg. 3959, 1994. |
| man | TDLo | oral | 10mg/kg/2W-I (10mg/kg) | BLOOD: HEMORRHAGE SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Archives of Internal Medicine. Vol. 158, Pg. 191, 1998. |
| mouse | LDLo | intraperitoneal | 400mg/kg (400mg/kg) | BEHAVIORAL: TREMOR LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Kiso to Rinsho. Clinical Report. Vol. 28, Pg. 3959, 1994. |
| mouse | LDLo | oral | 1gm/kg (1000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: ATAXIA | Kiso to Rinsho. Clinical Report. Vol. 28, Pg. 3959, 1994. |
| rat | LDLo | intraperitoneal | 200mg/kg (200mg/kg) | BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Kiso to Rinsho. Clinical Report. Vol. 28, Pg. 3959, 1994. |
| rat | LDLo | oral | 2gm/kg (2000mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Kiso to Rinsho. Clinical Report. Vol. 28, Pg. 3959, 1994. |
| women | TDLo | oral | 1mg/kg/1D-I (1mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" | Archives of Internal Medicine. Vol. 158, Pg. 2063, 1998. |
Production of Losartan potassium:
2-butyl-4-chloro-5-(hydroxymethyl) -1-[[2'-(triphenylmethyl) tetrazole -5-yl] biphenyl-4-yl] methyl] imidazole can be used to manufacture Losartan potassium under the condition of Hydrochloric acid and THF. The detailed steps are as follows:
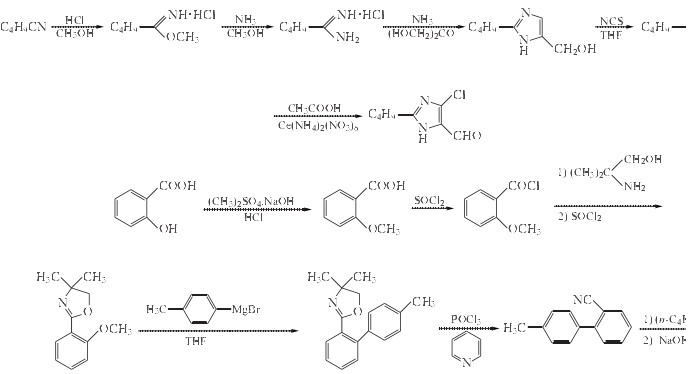
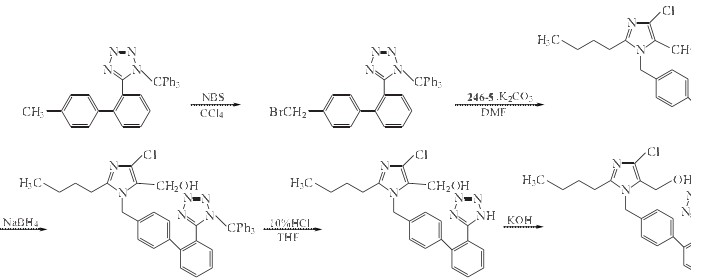
Related Products
- Losartan
- Losartan carboxylic acid
- Losartan potassium
- 124751-10-6
- 124751-11-7
- 124752-23-4
- 124760-64-1
- 124763-51-5
- 124-76-5
- 1247-69-4
- 124772-05-0
- 124774-36-3
- 124774-48-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View