-
Name
Mangiferin
- EINECS 624-757-7
- CAS No. 4773-96-0
- Article Data7
- CAS DataBase
- Density 1.843 g/cm3
- Solubility
- Melting Point 269-270°C
- Formula C19H18O11
- Boiling Point 842.7 °C at 760 mmHg
- Molecular Weight 422.345
- Flash Point 303.6 °C
- Transport Information UN 3462 6.1/PG 1
- Appearance Light yellow powder
- Safety 22-28-36/37-45-36-26
- Risk Codes 28-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T+,
T+, N
N
- Synonyms Mangiferin(6CI,7CI,8CI);1,3,6,7-Tetrahydroxyxanthone-C2-b-D-glucoside;2-C-b-D-Glucopyranosyl-1,3,6,7-tetrahydroxyxanthone;Alpizarin;Alpizarine;Aphloiol;Chinomin;Chinonin;Hedysarid;NSC 248870;Shamimin;
- PSA 201.28000
- LogP -0.71650
Synthetic route
-
-
1239694-50-8
2-C-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyl)-6-methoxy-1,3,7-trihydroxyxanthone

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 0 - 20℃; | 94% |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With 10% palladium hydroxide on charcoal; hydrogen In methanol at 20℃; under 760.051 Torr; for 48h; | 90% |
-
-
92631-83-9
3-glucosyl-2,3′,4,4′,6-pentahydroxybenzophenone

-
A
-
24699-16-9
4-β-D-glucopyranosyl-1,3-6,7-tetrahydroxy-9H-xanthen-9-one

-
B
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With sodium carbonate; potassium hexacyanoferrate(III) In water for 1.5h; Ambient temperature; | A 12 mg B 7 mg |
| With sodium carbonate; potassium hexacyanoferrate(III) In water for 1.5h; Product distribution; Ambient temperature; | A 12 mg B 7 mg |

| Conditions | Yield |
|---|---|
| In water at 37℃; tannase; |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 100℃; for 1h; Product distribution; other reagent (2N NaOH); other temp.; |
-
-
92665-82-2
maclurin 3-C-(6''-O-p-hydroxybenzoyl)-β-D-glucoside

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 20 mg / H2O / 35 °C / hesperidinase 2: 7 mg / K3, Na2CO3 / H2O / 1.5 h / Ambient temperature View Scheme |
-
-
92631-85-1
maclurin 3-C-(2''-O-p-hydroxybenzoyl-6''-O-galloyl)-β-D-glucoside

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 40 mg / H2O / 37 °C / hesperidinase 2: 7 mg / K3, Na2CO3 / H2O / 1.5 h / Ambient temperature View Scheme |
-
-
92631-84-0
maclurin 3-C-(2''-O-galloyl-6''-O-p-hydroxybenzoyl)-β-D-glucoside

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2O / 0.5 h / 37 °C / tannase 2: 20 mg / H2O / 35 °C / hesperidinase 3: 7 mg / K3, Na2CO3 / H2O / 1.5 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| In ethanol at 25℃; for 2h; Kinetics; Wavelength; |
-
-
92631-83-9
3-glucosyl-2,3′,4,4′,6-pentahydroxybenzophenone

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With ethylenediaminetetraacetic acid; NADPH at 30℃; for 2h; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: UDP; Mangifera indica C-glycosyltransferase / 12 h / Enzymatic reaction 2: NADPH; ethylenediaminetetraacetic acid / 2 h / 30 °C / Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Mangifera indica C-glycosyltransferase / 12 h / Enzymatic reaction 2: NADPH; ethylenediaminetetraacetic acid / 2 h / 30 °C / Enzymatic reaction View Scheme |
-
-
4291-69-4, 6386-24-9, 6564-72-3, 59531-24-7, 69257-52-9, 78184-89-1, 78609-16-2, 78609-17-3, 78609-18-4, 96553-53-6, 104111-61-7, 131347-08-5, 4132-28-9
2,3,4,6-Tetra-O-benzyl-D-glucopyranose

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: pyridine / 12 h / 20 °C 2.1: trimethylsilyl trifluoromethanesulfonate / dichloromethane; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 3.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 4.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 4.2: 1 h / -40 °C / Inert atmosphere 5.1: manganese(IV) oxide / dichloromethane / 36 h 6.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 7.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 8.1: caesium carbonate / methanol / 12 h / 80 °C 9.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 10.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1.1: boron trifluoride diethyl etherate / dichloromethane / 12 h / 20 °C / Inert atmosphere 2.1: sodium methylate / methanol / 1 h / 20 °C / pH 11 - 12 3.1: sodium hydride / N,N-dimethyl-formamide / 12 h / 0 - 20 °C 4.1: N-Bromosuccinimide; water / acetone / 3 h / 20 °C / Darkness 5.1: pyridine / 12 h / 20 °C 6.1: trimethylsilyl trifluoromethanesulfonate / dichloromethane; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 7.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 8.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 8.2: 1 h / -40 °C / Inert atmosphere 9.1: manganese(IV) oxide / dichloromethane / 36 h 10.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 11.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 12.1: caesium carbonate / methanol / 12 h / 80 °C 13.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 14.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |
-
-
80300-30-7
1-O-acetyl-2,3,4,6-tetra-O-benzyl-D-glucopyranose

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: trimethylsilyl trifluoromethanesulfonate / dichloromethane; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 2.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 3.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 3.2: 1 h / -40 °C / Inert atmosphere 4.1: manganese(IV) oxide / dichloromethane / 36 h 5.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 6.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 7.1: caesium carbonate / methanol / 12 h / 80 °C 8.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 9.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 12.5 h / 0 - 20 °C 2.1: N-Bromosuccinimide / N,N-dimethyl-formamide / 12 h / 20 °C 3.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 3.2: 1 h / -40 °C / Inert atmosphere 4.1: manganese(IV) oxide / dichloromethane / 36 h 5.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 6.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 7.1: caesium carbonate / methanol / 12 h / 80 °C 8.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 9.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |
-
-
156862-15-6
1,2,4-tris(methoxymethoxy)benzene

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: N-Bromosuccinimide / N,N-dimethyl-formamide / 12 h / 20 °C 2.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 2.2: 1 h / -40 °C / Inert atmosphere 3.1: manganese(IV) oxide / dichloromethane / 36 h 4.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 5.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 6.1: caesium carbonate / methanol / 12 h / 80 °C 7.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 8.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 1.2: 1 h / -40 °C / Inert atmosphere 2.1: manganese(IV) oxide / dichloromethane / 36 h 3.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 4.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 5.1: caesium carbonate / methanol / 12 h / 80 °C 6.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 7.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |
-
-
115130-39-7
1,3,5-tri-O-benzyl-2-C-(2,3,4,6-tetra-O-benzyl-β-D-glucopyranosyl)benzene

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 2.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 2.2: 1 h / -40 °C / Inert atmosphere 3.1: manganese(IV) oxide / dichloromethane / 36 h 4.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 5.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 6.1: caesium carbonate / methanol / 12 h / 80 °C 7.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 8.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |
-
-
28244-94-2, 28244-99-7, 86782-41-4, 131488-65-8
p-tolyl-2,3,4,6-tetra-O-acetyl-1-thio-β-D-glucopyranoside

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: sodium methylate / methanol / 1 h / 20 °C / pH 11 - 12 2.1: sodium hydride / N,N-dimethyl-formamide / 12 h / 0 - 20 °C 3.1: N-Bromosuccinimide; water / acetone / 3 h / 20 °C / Darkness 4.1: pyridine / 12 h / 20 °C 5.1: trimethylsilyl trifluoromethanesulfonate / dichloromethane; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 6.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 7.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 7.2: 1 h / -40 °C / Inert atmosphere 8.1: manganese(IV) oxide / dichloromethane / 36 h 9.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 10.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 11.1: caesium carbonate / methanol / 12 h / 80 °C 12.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 13.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 1.2: 1 h / -40 °C / Inert atmosphere 2.1: manganese(IV) oxide / dichloromethane / 36 h 3.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 4.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 5.1: caesium carbonate / methanol / 12 h / 80 °C 6.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 7.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: manganese(IV) oxide / dichloromethane / 36 h 2: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 3: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 4: caesium carbonate / methanol / 12 h / 80 °C 5: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 6: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 2: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 3: caesium carbonate / methanol / 12 h / 80 °C 4: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 5: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 2: caesium carbonate / methanol / 12 h / 80 °C 3: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 4: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: caesium carbonate / methanol / 12 h / 80 °C 2: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 3: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 2: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: sodium hydride / N,N-dimethyl-formamide / 12 h / 0 - 20 °C 2.1: N-Bromosuccinimide; water / acetone / 3 h / 20 °C / Darkness 3.1: pyridine / 12 h / 20 °C 4.1: trimethylsilyl trifluoromethanesulfonate / dichloromethane; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 6.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 6.2: 1 h / -40 °C / Inert atmosphere 7.1: manganese(IV) oxide / dichloromethane / 36 h 8.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 9.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 10.1: caesium carbonate / methanol / 12 h / 80 °C 11.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 12.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |
-
-
131531-76-5
p-methylphenyl 2,3,4,6-tetra-O-benzyl-thio-β-D-glucopyranoside

-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: N-Bromosuccinimide; water / acetone / 3 h / 20 °C / Darkness 2.1: pyridine / 12 h / 20 °C 3.1: trimethylsilyl trifluoromethanesulfonate / dichloromethane; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 4.1: trichlorophosphate / 16 h / 20 °C / Inert atmosphere 5.1: n-butyllithium / tetrahydrofuran / 1 h / -40 °C / Inert atmosphere 5.2: 1 h / -40 °C / Inert atmosphere 6.1: manganese(IV) oxide / dichloromethane / 36 h 7.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 8.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 2 h / 0 °C 9.1: caesium carbonate / methanol / 12 h / 80 °C 10.1: toluene-4-sulfonic acid / methanol / 0.5 h / 80 °C 11.1: 10% palladium hydroxide on charcoal; hydrogen / methanol / 48 h / 20 °C / 760.05 Torr View Scheme |

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid at 120℃; for 3.5h; | 93.6% |
-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With calcium hydroxide In dimethyl sulfoxide; glycerol Product distribution / selectivity; | 90.4% |
| With potassium hydrogencarbonate In ethanol; water Product distribution / selectivity; | 82.1% |
-
-
4773-96-0
mangiferin

-
-
929635-04-1
mangiferin-3-monosodium

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol; water Product distribution / selectivity; | 81.7% |
-
-
4773-96-0
mangiferin

-
-
1158716-92-7
mangiferin-3-monopotassium

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water Product distribution / selectivity; | 78.1% |
-
-
108-24-7
acetic anhydride

-
-
4773-96-0
mangiferin

-
-
4706-56-3
1,3,6,7-tetra-O-acetyl-2-C-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)xanthone

| Conditions | Yield |
|---|---|
| With iodine at 130℃; for 0.25h; Microwave irradiation; | 78% |
| With pyridine at 20℃; for 36h; |
-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| Stage #1: mangiferin With sodium hydrogencarbonate In water; dimethyl sulfoxide at 85℃; Stage #2: With calcium chloride In water; dimethyl sulfoxide at 20℃; Reagent/catalyst; | 74.5% |
-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; dimethyl sulfoxide at 60℃; Temperature; | 60.2% |
-
-
4773-96-0
mangiferin

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; dimethyl sulfoxide at 85℃; Temperature; Reagent/catalyst; | 50.5% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 65℃; for 5h; pH=7.5; | 46% |


| Conditions | Yield |
|---|---|
| With hydrogen iodide; phenol for 7h; Heating; | 43% |
| With hydrogenchloride; recorcinol In water for 6h; Reagent/catalyst; Reflux; | |
| With hydrogenchloride; recorcinol In water at 150℃; for 6h; Reagent/catalyst; Green chemistry; |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; ethanol at 65℃; for 6h; pH=7.5; | 40% |


| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 20h; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 168h; |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 20h; |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 600h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 43 percent / phenol, hydroiodic acid (d= 1.7) / 7 h / Heating 2: pyridine / 24 h View Scheme |
-
-
584-08-7
potassium carbonate

-
-
4773-96-0
mangiferin

-
A
-
1158716-92-7
mangiferin-3-monopotassium

-
B
-
1158716-92-7
mangiferin-7-monopotassium

| Conditions | Yield |
|---|---|
| In methanol; water Product distribution / selectivity; |

-
-
144-55-8
sodium hydrogencarbonate

-
-
4773-96-0
mangiferin

-
A
-
929635-04-1
mangiferin-3-monosodium

-
B
-
929635-04-1
mangiferin-7-monosodium

| Conditions | Yield |
|---|---|
| In ethanol; water Product distribution / selectivity; |

-
-
497-19-8
sodium carbonate

-
-
4773-96-0
mangiferin

-
A
-
929635-04-1
mangiferin-3-monosodium

-
B
-
929635-04-1
mangiferin-7-monosodium

| Conditions | Yield |
|---|---|
| In ethanol; water Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide; glycerol Product distribution / selectivity; |
Mangiferin Chemical Properties
Molecular Formula: C19H18O11
Molar mass: 422.34 g/mol
EINECS: 223-096-8
Density: 1.843 g/cm3
Flash Point: 303.6 °C
Index of Refraction: 1.788
Boiling Point: 842.7 °C at 760 mmHg
Vapour Pressure: 3.67E-30 mmHg at 25°C
Melting point: 269-270°C
Product categories of Mangiferin (4773-96-0): Xanthones;The group of Danshen
Structure of Mangiferin (4773-96-0):
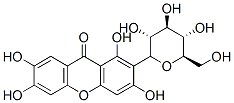
XLogP3-AA: -0.4
H-Bond Donor: 8
H-Bond Acceptor: 11
Systematic Name: 1,3,6,7-Tetrahydroxy-2-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]xanthen-9-one
SMILES: O=C1c3c(O)c(c(O)cc3Oc2cc(O)c(O)cc12)[C@@H]4O[C@@H]([C@@H](O)[C@H](O)[C@H]4O)CO
InChI: InChI=1/C19H18O11/c20-4-11-15(25)17(27)18(28)19(30-11)12-8(23)3-10-13(16(12)26)14(24)5-1-6(21)7(22)2-9(5)29-10/h1-3,11,15,17-23,25-28H,4H2/t11-,15-,17+,18-,19+/m1/s1
InChIKey: AEDDIBAIWPIIBD-ZJKJAXBQBF
Std. InChI: InChI=1S/C19H18O11/c20-4-11-15(25)17(27)18(28)19(30-11)12-8(23)3-10-13(16(12)26)14(24)5-1-6(21)7(22)2-9(5)29-10/h1-3,11,15,17-23,25-28H,4H2/t11-,15-,17+,18-,19+/m1/s1
Std. InChIKey: AEDDIBAIWPIIBD-ZJKJAXBQSA-N
Mangiferin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 2300ug/kg (2.3mg/kg) | Problemi na Vutreshnata Meditsina. Problems of Internal Medicine. Vol. 8(2), Pg. 109, 1980. | |
| rat | LD50 | intraperitoneal | 365mg/kg (365mg/kg) | Journal of Pharmaceutical Sciences. Vol. 61, Pg. 1838, 1972. |
Mangiferin Safety Profile
Hazard Codes: T+![]() ,Xi
,Xi![]()
Risk Statements:
28: Very Toxic if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
Mangiferin Specification
Mangiferin (4773-96-0) is belong to the group of Anemarrhenae, and also can be called Alpizarin ; 9H-Xanthen-9-one, 2-.beta.-D-glucopyranosyl-1,3,6,7-tetrahydroxy- ; D-glucitol, 1,5-anhydro-1-C-(1,3,6,7-tetrahydroxy-9-oxo-9H-xanthen-2-yl)-, (1S)- ;and (1S)-1,5-Anhydro-1-(1,3,6,7-tetrahydroxy-9-oxo-9H-xanthen-2-yl)-D-glucitol .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View