-
Name
Methyl 3,3-dimethylpent-4-enoate
- EINECS 264-431-8
- CAS No. 63721-05-1
- Article Data7
- CAS DataBase
- Density 0.893 g/cm3
- Solubility
- Melting Point
- Formula C8H14O2
- Boiling Point 136.3 °C at 760 mmHg
- Molecular Weight 142.198
- Flash Point 40.4 °C
- Transport Information UN 3272
- Appearance Colorless transparen tliquid
- Safety 16
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms 3,3-Dimethyl-4-pentenoicacid methyl ester;Penten-4-oic acid, 3,3-dimethyl, methyl ester;
- PSA 26.30000
- LogP 1.76170
Synthetic route
-
-
1445-45-0
Trimethyl orthoacetate

-
-
115-18-4
2-methyl-3-buten-2-ol

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With phosphoric acid at 190℃; under 9750.98 Torr; for 20h; Temperature; Claisen Rearrangement; | 97.2% |
-
-
1445-45-0
Trimethyl orthoacetate

-
-
556-82-1
3-methyl-2-buten-1-ol

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With phosphoric acid | 82.8% |
| With propionic acid at 145℃; for 2h; | 81% |
| With oenanthic acid at 180℃; | 80% |
-
-
74866-35-6
2-(1,1-dimethylallyl)malonic acid dimethyl ester

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With sodium cyanide In water; dimethyl sulfoxide at 160℃; | 60% |
-
-
70908-42-8
α,α-dimethyl allyl methyl carbonate

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: BSA / Mo(CO)6 / toluene / 100 °C 2: 60 percent / NaCN / dimethylsulfoxide; H2O / 160 °C View Scheme |

| Conditions | Yield |
|---|---|
| With B(C6F5)3 In methanol |

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
53589-56-3
3,3-dimethyl-4-pentenol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 0.166667h; Reduction; | 100% |
| With lithium aluminium tetrahydride In diethyl ether for 3h; Reflux; | 95% |
| Stage #1: methyl 3,3-dimethyl-4-penteneoate With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 1h; Stage #2: With water; sodium sulfate In tetrahydrofuran at 0℃; | 93% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
7796-73-8
3,3-dimethylpent-4-enoic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3,3-dimethyl-4-penteneoate With sodium hydroxide In water Reflux; Stage #2: With hydrogenchloride In water at 20℃; | 100% |
| Stage #1: methyl 3,3-dimethyl-4-penteneoate With sodium hydroxide for 5h; Reflux; Stage #2: With hydrogenchloride In water | 90.6% |
| With potassium hydroxide; sulfuric acid In ethanol | 77% |
-
-
354-58-5
1,1,1-Trichloro-2,2,2-trifluoroethane

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
72714-62-6
3,3-dimethyl-4,6,6-trichloro-7,7,7-trifluoro-enanthic acid methyl ester

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In tert-butyl alcohol at 115℃; under 2625.26 Torr; Reagent/catalyst; Temperature; | 99% |
| With tert-butyl peroxyneodecanoate at 60℃; for 6h; Reagent/catalyst; Inert atmosphere; Autoclave; Green chemistry; | 99.2% |
| With ethanolamine; copper(l) chloride In tert-butyl alcohol for 12h; Inert atmosphere; Reflux; Large scale; | 93.5% |
| Stage #1: 1,1,1-Trichloro-2,2,2-trifluoroethane With ethanolamine; copper(l) chloride In tert-butyl alcohol at 80℃; for 0.666667h; Inert atmosphere; Autoclave; Stage #2: methyl 3,3-dimethyl-4-penteneoate In tert-butyl alcohol under 3000.3 Torr; for 14h; Inert atmosphere; Autoclave; |
-
-
558-13-4
carbon tetrabromide

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide at 80℃; for 6h; Inert atmosphere; Autoclave; Green chemistry; | 98.3% |

| Conditions | Yield |
|---|---|
| With diacetyl peroxide at 80℃; for 6h; Inert atmosphere; Autoclave; Green chemistry; | 98.1% |

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3,3-dimethyl-4-penteneoate With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78 - 0℃; for 0.5h; Inert atmosphere; Stage #2: 1-Bromo-2-butyne In tetrahydrofuran; hexane at -40℃; for 0.75h; | 97% |
-
-
56-23-5
tetrachloromethane

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
64667-33-0
methyl 4,6,6,6-tetrachloro-3,3-dimethylhexanoate

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide at 80℃; for 8h; | 95% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
173932-32-6
3,3,5-trimethyl-5-hydroxy-1-hexene

| Conditions | Yield |
|---|---|
| With methylmagnesium bromide; ammonium chloride In diethyl ether | 95% |
-
-
21032-48-4
2-(dimethylhydrosilyl)pyridine

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
226225-90-7
dimethyl(4-methoxycarbonyl-3,3-dimethylpentyl)(2-pyridyl)silane

| Conditions | Yield |
|---|---|
| RhCl(PPh3)3 In acetonitrile for 1h; Ambient temperature; | 83% |
| With RhCl(PPh3)3 In acetonitrile at 20℃; for 0.5h; | 83% |

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In toluene | 82% |

| Conditions | Yield |
|---|---|
| With 2,2'-bis((diphenylphosphino)methyl)-1,1'-biphenyl; dicarbonylacetylacetonato rhodium (I); C29H32ClIrNO; hydrogen; sodium formate In methanol; toluene at 80℃; under 2550.26 Torr; for 20h; regioselective reaction; | 82% |

-
-
123820-43-9, 123820-44-0, 84393-12-4
[(1-methoxy-2-methyl-1-butenyl)oxy]trimethylsilane

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 20 - 25℃; for 1h; Claisen condensation; Inert atmosphere; | A 81% B 6% |
| With sodium hydroxide In N,N-dimethyl-formamide at 15 - 20℃; for 1h; crossed Claisen condensation; |

-
-
4406-72-8
2-phenyl[1,3,2]dioxaborolane

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) bis(trifluoromethanesulfonate) In N,N-dimethyl acetamide at 40℃; under 760.051 Torr; for 48h; Heck Reaction; regioselective reaction; | 81% |
-
-
64-18-6
formic acid

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With carbon monoxide; palladium on activated charcoal; 1,4-di(diphenylphosphino)-butane In 1,2-dimethoxyethane at 150℃; under 5168 Torr; for 24h; | 80% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With 8; Speier's catalyst In hexane for 48h; Heating; | 80% |
-
-
554-14-3
2-Methylthiophene

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With sodium 3-(methylthio)propane-1-sulfonate; palladium diacetate; acetic acid; hydroquinone at 40℃; for 18h; | 79% |
| With palladium diacetate; copper(II) acetate monohydrate In dimethyl sulfoxide for 0.5h; Heck Reaction; Milling; chemoselective reaction; | 40% |
-
-
354-51-8
1,2-dibromo-1-chloro-1,2,2-trifluoroethane

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
141-43-5
ethanolamine

-
-
89608-40-2
methyl 3,3-dimethyl-4,7-dibromo-6-chloro-6,7,7-trifluoroheptanoate

| Conditions | Yield |
|---|---|
| 78% |

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
78984-87-9
methyl 4,5-epoxy-3,3-dimethylpentanoate

| Conditions | Yield |
|---|---|
| With rhenium trioxide; bis-trimethylsilanyl peroxide; water In dichloromethane at 20℃; for 15h; | 77% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With trichlorosilane; dihydrogen hexachloroplatinate | 77% |
-
-
940867-32-3
2-(tert-butyldimethylsilyloxy)-1-methoxy-1-trimethylsilyloxy-1-propene

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
1178908-68-3
methyl 2-hydroxy-2,5,5-trimethyl-3-oxohept-6-enoate

| Conditions | Yield |
|---|---|
| Stage #1: 2-(tert-butyldimethylsilyloxy)-1-methoxy-1-trimethylsilyloxy-1-propene; methyl 3,3-dimethyl-4-penteneoate With sodium hydroxide In 1-methyl-pyrrolidin-2-one at 20 - 25℃; for 3h; Claisen condensation; Inert atmosphere; Stage #2: With tetrabutyl ammonium fluoride In tetrahydrofuran at 0 - 5℃; for 1h; Inert atmosphere; | 76% |
-
-
23542-51-0
5-nitropent-1-ene

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane for 18h; Heating; | 73% |
-
-
1392314-08-7
(1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-amino-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
1622928-60-2
methyl 5-((1R,3aS,5aR,5bR,7aR,11aS,11bR,13aR,13bR)-3a-amino-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl)-3,3-dimethylpentanoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3,3-dimethyl-4-penteneoate With 9-bora-bicyclo[3.3.1]nonane In tetrahydrofuran at 0 - 20℃; for 2h; Inert atmosphere; Cooling with ice; Stage #2: (1R,3aS,5aR,5bR,7aR,11aR,11bR,13aR,13bR)-3a-amino-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl trifluoromethanesulfonate With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium phosphate; phosphoric acid In tetrahydrofuran; 1,4-dioxane; toluene at 85℃; for 16h; Temperature; Time; Inert atmosphere; | 72.8% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
2719-27-9
cyclohexanylcarbonyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3,3-dimethyl-4-penteneoate; cyclohexanylcarbonyl chloride With 1-methyl-1H-imidazole In dichloromethane at -45℃; for 0.166667h; Stage #2: With tributyl-amine; titanium tetrachloride In dichloromethane at -45℃; for 0.5h; | 69% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In 1,4-dioxane at 90℃; for 12h; Inert atmosphere; | 68% |
-
-
144-62-7
oxalic acid

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With carbon monoxide; palladium on activated charcoal; 1,4-di(diphenylphosphino)-butane In 1,2-dimethoxyethane at 150℃; under 30400 Torr; for 24h; | 67% |

-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; magnesium(II) acetate tetrahydrate; 4,4'-di-tert-butyl-2,2'-bipyridine In N,N-dimethyl acetamide at 40℃; for 2h; Schlenk technique; Inert atmosphere; regioselective reaction; | 67% |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
106-96-7
propargyl bromide

-
-
119548-51-5
methyl 3,3-dimethylhepta-1-ene-6-yne-4-carboxylate

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; | 66% |
| With lithium diisopropyl amide 1) THF, HMPA, -63 deg C, 15 min, 2) THF, HMPA, -63 deg C, 20 min.; Yield given. Multistep reaction; |
-
-
63721-05-1
methyl 3,3-dimethyl-4-penteneoate

-
-
98-80-6
phenylboronic acid

| Conditions | Yield |
|---|---|
| With palladium diacetate; 2,3-dicyano-5,6-dichloro-p-benzoquinone; trichloroacetic acid In neat (no solvent) for 0.5h; Heck Reaction; Milling; chemoselective reaction; | 65% |
| With palladium diacetate; 2,3-dicyano-5,6-dichloro-p-benzoquinone; trichloroacetic acid for 0.666667h; Heck Reaction; Milling; Sonication; |
Methyl 3,3-dimethylpent-4-enoate Specification
The CAS registry number of 4-Pentenoic acid,3,3-dimethyl-, methyl ester is 63721-05-1. With the EINECS registry number 264-431-8, its systematic name is methyl 3,3-dimethylpent-4-enoate. In addition, the molecular formula is C8H14O2 and the molecular weight is 142.20. What's more, it is a colorless clear liquid and should be stored in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 2.15; (2)ACD/LogD (pH 5.5): 2.15; (3)ACD/LogD (pH 7.4): 2.15; (4)ACD/BCF (pH 5.5): 25.37; (5)ACD/BCF (pH 7.4): 25.37; (6)ACD/KOC (pH 5.5): 352.24; (7)ACD/KOC (pH 7.4): 352.24; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 4; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.423; (12)Molar Refractivity: 40.57 cm3; (13)Molar Volume: 159.2 cm3; (14)Polarizability: 16.08 ×10-24cm3; (15)Surface Tension: 25.9 dyne/cm; (16)Density: 0.893 g/cm3; (17)Flash Point: 40.4 °C; (18)Enthalpy of Vaporization: 37.37 kJ/mol; (19)Boiling Point: 136.3 °C at 760 mmHg; (20)Vapour Pressure: 7.43 mmHg at 25°C.
Preparation of 4-Pentenoic acid,3,3-dimethyl-, methyl ester: it can be prepared by 3-methyl-2-butenol and trimethyl orthoacetate in the presence of catalyst phenol. Add these raw materials into the reactor with stirring. Then heat the mixture to 95 °C and react for 2-3 hours. Next, heat the reaction solution to 140 °C in an hour and react for 20 hours. After this reaction, you should collect 70-71 °C(8.00kPa) fractions through vacuum distillation.
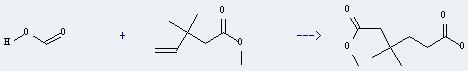
Uses of 4-Pentenoic acid,3,3-dimethyl-, methyl ester: it can react with formic acid to get 3,3-dimethyl-hexanedioic acid 1-methyl ester. This reaction will need reagent CO, catalysts 10 percent Pd/C and dppb and solvent 1,2-dimethoxy-ethane. The reaction time is 24 hours at reaction temperature of 150 °C. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable. You should keep it away from sources of ignition - No smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC)CC(/C=C)(C)C
(2)Std. InChI: InChI=1S/C8H14O2/c1-5-8(2,3)6-7(9)10-4/h5H,1,6H2,2-4H3
(3)Std. InChIKey: MKLKDUHMZCIBSJ-UHFFFAOYSA-N
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 6372-14-1
- 63721-83-5
- 6372-40-3
- 6372-42-5
- 63725-51-9
- 6372-96-9
- 63731-07-7
- 6373-11-1
- 6373-14-4
- 637318-31-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View