-
Name
Methyl 3-oxovalerate
- EINECS 250-184-3
- CAS No. 30414-53-0
- Article Data32
- CAS DataBase
- Density 1.015 g/cm3
- Solubility slightly soluble in water
- Melting Point -35 °C
- Formula C6H10O3
- Boiling Point 207.8 °C at 760 mmHg
- Molecular Weight 130.144
- Flash Point 71.1 °C
- Transport Information
- Appearance clear colorless to slightly yellow liquid
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xi
Xi
- Synonyms Valericacid, 3-oxo-, methyl ester (7CI,8CI);3-Oxopentanoic acid methyl ester;3-Oxovaleric acid methyl ester;Methyl 3-oxopentanoate;Methyl propanoylacetate;Methyl propionylacetate;Methyl b-ketovalerate;
- PSA 43.37000
- LogP 0.52860
Synthetic route

| Conditions | Yield |
|---|---|
| With n-butyllithium; sodium hydride In tetrahydrofuran; ethanol at 20℃; for 3h; | 97% |
| (i) NaH, THF, (ii) nBuLi, hexane, (iii) /BRN= 969135/, aq. HCl, Et2O; Multistep reaction; | |
| With n-butyllithium; sodium hydride 1) THF, 0 deg C, 20 min, 2) THF, room temperature, 30 min; Yield given. Multistep reaction; |
-
-
67-56-1
methanol

-
-
64074-05-1
2,2-dimethyl-5-propanoyl-1,3-dioxan-4,6-dione

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| for 0.0666667h; microwave irradiation; | 90% |
| at 80℃; for 3h; Reflux; |
-
-
67-56-1
methanol

-
-
66696-76-2
5-(1-hydroxypropylidene)-2,2-dimethyl-[1,3]dioxane-4,6-dione

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| Heating; | 87% |
-
-
24342-04-9
methyl pent-2-ynoate

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With silica-supported HgSO4/H2SO4/H2O In dichloromethane at 40℃; for 5h; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: carbonic acid dimethyl ester With sodium hydride at 20℃; Inert atmosphere; Stage #2: butanone for 4h; Inert atmosphere; Reflux; | 75% |
| Stage #1: carbonic acid dimethyl ester With sodium hydride In toluene Reflux; Inert atmosphere; Stage #2: butanone In toluene Reflux; Inert atmosphere; | |
| With sodium hydride In toluene Inert atmosphere; Reflux; | |
| With sodium hydride In toluene Inert atmosphere; Reflux; |

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethyl-2-(2-methoxy-2-oxoethyl)malonic acid With ammonia In methanol at 20℃; Electrochemical reaction; Stage #2: With hydrogenchloride In methanol; water at 20℃; | 75% |
-
-
79-03-8
propionyl chloride

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With calcium hydroxide In dichloromethane; ammonia; butanone | 71% |

| Conditions | Yield |
|---|---|
| In diethyl ether for 60h; | 57% |
-
-
37517-81-0
3-chloro-3-oxopropanoic acid methyl ester

-
-
925-90-6
ethylmagnesium bromide

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: ethylmagnesium bromide With pentadec-1-yne In diethyl ether at 0 - 20℃; for 1.33333h; Inert atmosphere; Stage #2: 3-chloro-3-oxopropanoic acid methyl ester In diethyl ether at 0℃; for 0.666667h; Inert atmosphere; | 35% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
10191-25-0
3-oxo-valeric acid

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With diethyl ether |
-
-
20577-62-2
methyl 2,4-dioxohexanoate

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With glass powder at 240℃; | |
| Destillation ueber Glaspulver; |
-
-
883528-73-2
3-amino-4-methyl crotonic acid methylester

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
17448-81-6
ethyl 2-acetyl-3-oxopentanoate

-
-
124-41-4
sodium methylate

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| In methanol |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
78-93-3
butanone

-
A
-
17094-21-2
2-methyl-acetoacetic acid methyl ester

-
B
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| 1.) DMF, 110 deg C, 3 h, 2.) ether; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
7647-01-0
hydrogenchloride

-
-
883528-73-2
3-amino-4-methyl crotonic acid methylester

-
-
30414-53-0
methyl propanoyl acetate

-
-
79-03-8
propionyl chloride

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ammonia In tetrahydrofuran |
-
-
10191-25-0
3-oxo-valeric acid

-
-
30414-53-0
methyl propanoyl acetate
-
-
38330-80-2
monomethyl monopotassium malonate

-
-
802294-64-0
propionic acid

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole; magnesium chloride In tetrahydrofuran |
-
-
56009-31-5
3-hydroxy valeric acid methyl ester

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With Candida parapsilosis carbonyl reductase 2 WT; nicotinamide adenine dinucleotide In aq. buffer pH=8; Kinetics; Reagent/catalyst; Enzymatic reaction; |
-
-
66696-76-2
5-(1-hydroxypropylidene)-2,2-dimethyl-[1,3]dioxane-4,6-dione

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; |
-
-
30414-53-0
methyl propanoyl acetate

-
-
42558-50-9, 56009-31-5, 60793-22-8, 133098-13-2
methyl (3R)-3-hydroxypentanoate

| Conditions | Yield |
|---|---|
| With cat; hydrogen In methanol; dichloromethane at 25℃; under 77572.2 Torr; for 48h; | 100% |
| With [(R)-BINAP]RuBr2; hydrogen In methanol at 40℃; under 15200 Torr; for 16h; | 100% |
| With (S)-4,12-bis(diphenylphosphino)-<2.2>paracyclophane-Ru(II)bis(trifluoroacetate); hydrogen; tetra-(n-butyl)ammonium iodide In methanol; water at -5℃; under 2585.7 Torr; for 18h; | 100% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
42558-50-9
methyl (S)-3-hydroxyvalerate

| Conditions | Yield |
|---|---|
| With cat; hydrogen In methanol; dichloromethane at 25℃; under 77572.2 Torr; for 48h; | 100% |
| With (S)-BinapRuBr2; hydrogen In methanol at 40℃; under 15200 Torr; for 16h; | 100% |
| With baker's yeast In various solvent(s) at 20℃; for 24h; | 100% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
210691-47-7
methyl 2-benzyl-3-oxo-pentanoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl propanoyl acetate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 25℃; for 1h; Inert atmosphere; Stage #2: benzyl halide In tetrahydrofuran; mineral oil at 0℃; | 100% |
| With potassium carbonate In tetrahydrofuran Heating; | |
| Stage #1: methyl propanoyl acetate With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: benzyl halide In tetrahydrofuran; mineral oil Inert atmosphere; |
-
-
2916-68-9
2-(Trimethylsilyl)ethanol

-
-
30414-53-0
methyl propanoyl acetate

-
-
215120-12-0
trimethylsilyl 3-oxovaleric ester

| Conditions | Yield |
|---|---|
| With dmap In toluene Heating / reflux; | 100% |
-
-
41340-36-7
7-ethyl-2-(1H-indol-3-yl)ethan-1-ol

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In methanol at 20℃; for 22h; Reagent/catalyst; Temperature; | 99.9% |
| With sulfuric acid In methanol at 0 - 30℃; Reagent/catalyst; Pictet-Spengler Synthesis; | 98% |
-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: methyl propanoyl acetate With hydroxylamine hydrochloride; sodium acetate In ethanol Reflux; Stage #2: With hydrogenchloride In ethanol; water Reflux; | 99% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
100-34-5
benzene diazonium chloride

-
-
112031-44-4
methyl 2,3-dioxopentanoate 2-phenylhydrazone

| Conditions | Yield |
|---|---|
| 98% |

| Conditions | Yield |
|---|---|
| With Ni loaded silica In ethanol at 20℃; for 1.5h; | 98% |
| With boric acid; acetic acid at 100℃; for 7h; Biginelli reaction; | 23.59% |

-
-
30414-53-0
methyl propanoyl acetate

-
-
105983-77-5
methyl 4-bromo-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at 5℃; | 98% |
| With bromine; sodium carbonate In chloroform | 78% |
| With bromine In chloroform at 0 - 20℃; | 78% |

-
-
30414-53-0
methyl propanoyl acetate

-
-
42558-50-9, 56009-31-5, 60793-22-8, 133098-13-2
methyl (3R)-3-hydroxypentanoate

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane; nitrogen | 98% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
17356-08-0
thiourea

-
-
613-45-6
2,4-Dimethoxybenzaldehyde

-
-
1373955-95-3
6-ethyl-4-(2,4-dimethoxyphenyl)-5-(methoxycarbonyl)-3,4-dihydropyrimidin-2(1H)-thione

| Conditions | Yield |
|---|---|
| With Ni loaded silica In ethanol at 20℃; for 1.5h; | 98% |

-
-
30414-53-0
methyl propanoyl acetate

-
-
3952-66-7
methyl 2-oxobutanoate

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; Oxone In water at 20℃; for 0.166667h; | 98% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
1493-27-2
ortho-nitrofluorobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 92h; Inert atmosphere; | 98% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
17758-33-7
3-methyl-5-nitro-4(3H)-pyrimidinone

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol for 72h; Heating; | 97% |
-
-
5153-67-3
nitrostyrene

-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| [(S,S)-N-(pentamethylbenzenesulfonyl)-1,2-diphenylethylenediamine] (hexamethylbenzene)ruthenium at -20℃; for 48h; Michael Condensation; | 97% |
| With C28H33N5O2 In dichloromethane at 20℃; Michael condensation; enantioselective reaction; | 90% |
| With N21,N23-bis(4-bromobenzyl)-2-(((2-(pyridin-3-yl)ethyl)amino)methyl)-5,10,15,20-tetrakis(3,5-ditert-butyl-4-oxo-cyclohexa-2,5-dienylidene)porphyrinogen In ethanol at 20℃; for 16h; Michael Addition; Inert atmosphere; | 87% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
23326-27-4
Methyl 2-butynoate

-
-
107941-27-5
methyl 6-ethyl-4-methyl-2-oxo-2H-pyran-5-carboxylate

| Conditions | Yield |
|---|---|
| With sodium methylate at 100℃; for 3h; | 96% |
| With sodium methylate | 96% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
4346-59-2
para-methoxyphenyldiazonium chloride

| Conditions | Yield |
|---|---|
| With sodium acetate In ethanol Ambient temperature; | 96% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
74-88-4
methyl iodide

-
-
17422-12-7
methyl 2-methyl-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Inert atmosphere; Reflux; | 96% |
| Stage #1: methyl propanoyl acetate With potassium carbonate In acetone for 0.0833333h; Stage #2: methyl iodide In [(2)H6]acetone for 12h; Reflux; | 94% |
| Stage #1: methyl propanoyl acetate With potassium carbonate In tetrahydrofuran for 3h; Heating; Stage #2: methyl iodide In tetrahydrofuran cooling; | 92% |

| Conditions | Yield |
|---|---|
| With silica gel; zinc(II) chloride for 0.00555556h; microwave irradiation; | 96% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
114192-09-5
methyl 2-chloro-3-oxopentanoate

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane at 0 - 25℃; for 16h; | 96% |
| With sulfuryl dichloride In toluene | 131.6 g (0.8 mol; yield: 100%) |
| With sulfuryl dichloride In toluene | 131.6 g (0.8 mol; yield: 100%) |
| With sulfuryl dichloride In dichloromethane at 23℃; for 2h; | |
| With sulfuryl dichloride In dichloromethane at 20℃; for 4h; Cooling with ice; | 6 g |
-
-
621-59-0
isovanillin

-
-
30414-53-0
methyl propanoyl acetate

-
-
17356-08-0
thiourea

-
-
1373955-96-4
6-ethyl-4-(3-hydroxy-4-methoxyphenyl)-5-(methoxycarbonyl)-3,4-dihydro-pyrimidin-2(1H)-thione

| Conditions | Yield |
|---|---|
| With Ni loaded silica In ethanol at 20℃; for 1.5h; | 96% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 3h; Heating; | 95.7% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
10191-25-0
3-oxo-valeric acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 25℃; for 24h; | 95% |
| Stage #1: methyl propanoyl acetate With sodium hydroxide at 25℃; for 24h; Stage #2: With hydrogenchloride In water pH=2; | 95% |
| With sodium hydroxide; water | 67% |
-
-
30414-53-0
methyl propanoyl acetate

-
-
99-61-6
3-nitro-benzaldehyde

-
-
40035-23-2
2,6-Diethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridine-3,5-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With C23H3BF16N2O; ammonium acetate In toluene at 100℃; for 10h; Hantzsch Dihydropyridine Synthesis; | 95% |
| With ammonium hydroxide In methanol for 4h; Heating; | 59% |
-
-
30414-53-0
methyl propanoyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: methyl propanoyl acetate With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.75h; Inert atmosphere; Stage #2: With n-butyllithium In tetrahydrofuran; hexane; mineral oil at 0℃; for 0.666667h; Inert atmosphere; Stage #3: benzyl 2,3-anhydro-4-O-methoxymethyl-β-L-ribopyranoside In tetrahydrofuran; hexane; mineral oil at 0 - 20℃; Inert atmosphere; regioselective reaction; | 95% |
| With n-butyllithium; sodium hydride In tetrahydrofuran at 0 - 25℃; for 12h; |

| Conditions | Yield |
|---|---|
| indium(III) chloride In tetrahydrofuran for 6h; Condensation; cyclization; Heating; | 95% |
| With boron trifluoride diethyl etherate; acetic acid; copper(l) chloride In tetrahydrofuran at 65℃; | 81% |
| With sulfuric acid; copper(l) chloride In methanol Biginelli Pyrimidone Synthesis; Reflux; Inert atmosphere; | 71% |
| With hydrogenchloride In ethanol Biginelli reaction; Heating; | 13.5% |


| Conditions | Yield |
|---|---|
| With C23H3BF16N2O; ammonium acetate In toluene at 100℃; for 10h; Hantzsch Dihydropyridine Synthesis; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol; methyl propanoyl acetate; anthranilic acid amide With Candida antarctica lipase B at 60℃; for 60h; Enzymatic reaction; Stage #2: With α-chymotrypsin at 60℃; for 40h; Enzymatic reaction; | 95% |

-
-
30414-53-0
methyl propanoyl acetate

-
-
57-13-6
urea

| Conditions | Yield |
|---|---|
| With hydrogenchloride; iron(III) chloride hexahydrate In ethanol for 2h; Biginelli Pyrimidone Synthesis; Reflux; | 95% |

Methyl 3-oxovalerate Chemical Properties
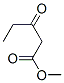
IUPAC Name:methyl 3-oxopentanoate
Molecular Formula:C6H10O3
Molecular Weight:130.141800 g/mol
Melting Point:-35 °C
Boiling Point:73-74 °C5 mm Hg(lit.)
Flash Point:71 °C
density:1.037 g/mL at 25 °C(lit.)
refractive index:n20/D 1.422(lit.)
Water Solubility:SLIGHTLY SOLUBLE
BRN:1753684
CAS DataBase Reference:30414-53-0(CAS DataBase Reference)
NIST Chemistry Reference:30414-53-0(NIST)
Synonyms of Methyl 3-oxovalerate(30414-53-0):
PROPIONYLACETIC ACID METHYL ESTER;PEM;MOP;3-oxo-methylvalerate;Methy3-oxo-valerate;Methyl 3-oxo-n-valerate;Methyl beta-ketovalerate;methyl3-0xopentanoate
Categories of Methyl 3-oxovalerate(30414-53-0):straight chain compounds
Methyl 3-oxovalerate Uses
Methyl 3-oxovalerate Safety Profile
Safety Information of Methyl 3-oxovalerate(30414-53-0):
Hazard Codes:F  ,Xi
,Xi 
Risk Statements:36/37/38
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:24/25-36-26
24/25:Avoid contact with skin and eyes
36:Wear suitable protective clothing
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany:3
Hazard Note:Flammable
HS Code:29183000
Related Products
- Methyl (2R)-2-bromo-2-(2-chlorophenyl)acetate
- Methyl (2R,3S)-3-(4-methoxyphenyl)-2-oxiranecarboxylate
- Methyl (2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
- Methyl (3R,4S)-1-(4-fluorophenyl)-2-oxo-4-[4-(phenylmethoxy)phenyl]-3-azetidinepropanoate
- Methyl [(dimethoxyphosphinothioyl)thio]acetate
- Methyl [6,6]-phenyl-C61-butyrate
- Methyl 1-benzyl-4-((propionyl)phenylamino)piperidine-4-carboxylate
- Methyl 1-benzyl-4-(4-methoxyphenyl)pyrrolidine-3-carboxylate
- Methyl 1-benzyl-4-methylpyrrolidine-3-carboxylate
- 30414-54-1
- 304-17-6
- 30418-38-3
- 30418-59-8
- 3042-00-0
- 304-20-1
- 304-21-2
- 3042-81-7
- 30429-79-9
- 30431-54-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View