-
Name
Methyl phenylacetate
- EINECS 202-940-9
- CAS No. 101-41-7
- Article Data423
- CAS DataBase
- Density 1.055 g/cm3
- Solubility Miscible with water.
- Melting Point 218 °C(lit.)
- Formula C9H10O2
- Boiling Point 218 °C at 760 mmHg
- Molecular Weight 150.177
- Flash Point 93.9 °C
- Transport Information
- Appearance colourless liquid
- Safety 23-24/25
- Risk Codes 21
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Aceticacid, phenyl-, methyl ester (6CI,8CI);2-Methoxy-1-phenyl-2-oxoethane;Methylbenzeneacetate;Methyl benzeneethanoate;Methylphenylethanoate;Methyl a-phenylacetate;Methyl a-toluate;NSC 401667;NSC 9405;Phenylacetic acid methyl ester;Benzeneaceticacid, methyl ester;
- PSA 26.30000
- LogP 1.40210
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 1h; Heating; | 100% |
| With sulfuric acid for 4h; Reflux; | 100% |
| With sulfuric acid Heating; | 99% |

| Conditions | Yield |
|---|---|
| With tert-butylamine for 21h; Heating; | 100% |
| With indium; iodine for 5.5h; transesterification; Heating; | 86% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; lithium bromide for 5h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With tert-butylamine; lithium bromide for 0.25h; Heating; | 100% |
| With indium; iodine for 12h; transesterification; Heating; | 88% |
| In acetonitrile Ambient temperature; Et4NClO4 electrolyte, glassy carbon cathode, Pt anode, -1,7 V potential; | 43% |
| With PCS-DBU In water at 60℃; Rate constant; Mechanism; other quaternary ammonium resins, other temperatures, other reaction time; |
-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
-
100-39-0
benzyl bromide

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; C52H46O2P2Pd2; triphenylphosphine at 60℃; for 2h; Conversion of starting material; | 100% |
| With N-ethyl-N,N-diisopropylamine; C26H24BrOPPd; triphenylphosphine at 28 - 60℃; under 760.051 - 2587.76 Torr; for 2h; Conversion of starting material; | 99% |
| With N-ethyl-N,N-diisopropylamine; C43H37BrOP2Pd; triphenylphosphine at 60℃; for 2h; Conversion of starting material; | 99% |

-
-
107-31-3
Methyl formate

-
-
201230-82-2
carbon monoxide

-
-
100-39-0
benzyl bromide

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium iodide; <1,5-HDRhCl>2 under 5171.5 Torr; Ambient temperature; | 100% |


| Conditions | Yield |
|---|---|
| With tert-butylamine for 2h; Heating; | 100% |
| (EtO)3TiO(CH2)2OTi(OEt)3 for 72h; Heating; | 91% |
| scandium tris(trifluoromethanesulfonate) at 64℃; for 10h; | 91% |
| With Rhizobium meliloti cyclosophoraoses at 60℃; Kinetics; Enzyme kinetics; Further Variations:; Reaction partners; reaction time; |

| Conditions | Yield |
|---|---|
| With lithium perchlorate; methyl iodide Ambient temperature; anodic oxidation at constant current; | 100% |
| With poly[4-(diacetoxyiodo)styrene]; sulfuric acid In acetonitrile at 60℃; for 0.5h; | 80% |
| With lead(IV) acetate; perchloric acid at 50℃; for 2h; | 46% |
| With LiCO4*H2O; methyl iodide Product distribution; Mechanism; Ambient temperature; anodic oxidation at constatnt current; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In diethyl ether at 0℃; Inert atmosphere; | 100% |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
103-79-7
1-phenyl-acetone

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With Novozym 435; acylase I from Aspergillus melleus; amano lipase AK from pseudomonas fluorescens; lipase from wheat germ; papaine In toluene at 40℃; for 48h; Mechanism; Enzymatic reaction; | 100% |

| Conditions | Yield |
|---|---|
| With acid | 99% |
-
-
37167-62-7
bromo-phenyl-acetic acid methyl ester

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In ethanol at 80℃; for 0.00833333h; microwave irradiation; | 99% |
| With indium; acetic acid In methanol at 20℃; for 1h; | 97.6% |
| With dimethyl-3-pyrenylpropyl tin hydride; 2,2'-azobis(isobutyronitrile) In benzene for 1h; Heating; | 89% |
| With 2,2'-azobis(isobutyronitrile); dimethyl(3-(pyren-1-yl)propyl)stannane In benzene for 1h; Heating; | 89% |
| With sodium bis(2-methoxyethoxy)aluminium dihydride In toluene 1.) 10-20 deg C, 2.) room temperature, 10 min; | 80% |
-
-
103-82-2
phenylacetic acid

-
-
616-38-6
carbonic acid dimethyl ester

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 160℃; under 15001.2 Torr; for 0.2h; microwave irradiation; | 99% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene for 6h; Heating; | 98% |
| With sulfuric acid at 80 - 85℃; for 5.5h; Neat (no solvent); | 97.09% |
-
-
62054-83-5
(2-Iodo-1,1-dimethoxy-ethyl)-benzene

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In methanol at 20℃; for 5h; | 98% |
| In methanol for 4h; | 98% |
-
-
539814-10-3
4-[(methoxycarbonyl)methyl]phenyl mesylate

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With ammonium acetate; magnesium; palladium on activated charcoal In methanol at 20℃; for 12h; | 98% |
| With hydrogen; diethylamine; palladium on activated charcoal In methanol at 20℃; for 4h; | 89% |
| With ammonium acetate; methanol; magnesium; palladium on activated charcoal at 20℃; for 12h; | 98 % Spectr. |

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetonitrile at 80 - 85℃; for 16 - 18h; | 98% |
-
-
67-56-1
methanol

-
-
102-20-5
phenyl-acetic acid phenethyl ester

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium tetrachloridoferrate(III) at 146.84℃; for 6h; Reagent/catalyst; | 96.6% |
-
-
67-56-1
methanol

-
-
85909-01-9
Benzyl-phenylacetyl-carbamic acid tert-butyl ester

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium methylate at 0℃; for 0.416667h; | 96% |
-
-
86561-27-5
methyl O-acetylmandalate

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With methanol; N,N,N,N,N,N-hexamethylphosphoric triamide; samarium diiodide In tetrahydrofuran at 20 - 22℃; for 0.0166667h; | 96% |
| With sodium tetrahydroborate; nickel dichloride In methanol for 0.333333h; Ambient temperature; | 76% |
-
-
83994-44-9
trimethyl orthoselenophenylacetate

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With mercury dichloride; mercury(II) oxide In methanol; water for 5h; Heating; | 96% |
-
-
67-56-1
methanol

-
-
98-86-2
acetophenone

-
-
149-73-5
trimethyl orthoformate

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With bromine; silver nitrate for 108h; other oxidants; | 96% |

-
-
67-56-1
methanol

-
-
300680-47-1
1-[1-methoxy-2-phenylethenyl]-1H-1,2,3-benzotriazole

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride Heating; | 96% |
-
-
67-56-1
methanol

-
-
78323-99-6
tert-butyldimethylsilyl 2-phenylethanoate

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: methanol; tert-butyldimethylsilyl 2-phenylethanoate; carbon tetrabromide at 20℃; for 0.5h; Irradiation; Stage #2: at 20℃; for 2h; | 96% |

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In methanol at 20℃; for 12h; | 96% |

| Conditions | Yield |
|---|---|
| In methanol; water | 95.4% |
-
-
67-56-1
methanol

-
-
536-74-3
phenylacetylene

-
A
-
103-82-2
phenylacetic acid

-
B
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) for 48h; Yields of byproduct given; | A n/a B 95% |


| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 18h; Elimination; addition; Irradiation; | A 95% B 72% |

-
-
1179348-58-3
methyl 2-(dimethyl(oxo)-λ6-sulfaneylidene)-2-phenylacetate

-
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With water; isopropyl alcohol for 2h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide dimethyl acetal at 25℃; for 4h; | 94% |
| With potassium hydrogensulfate at 65℃; for 14h; | 93% |
| unter Einleiten von Borfluorid und Erwaermen des Reaktionsgemisches; |

| Conditions | Yield |
|---|---|
| With triethylamine; dmap In dichloromethane at 0℃; for 1h; | 94% |
| With dmap; triethylamine 1.) CH2Cl2, 0 deg C, 5 min, 2.) 0 deg C, 1 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| In acetonitrile at 35℃; under 8000 Torr; for 72h; | 100% |
| In acetonitrile at 35℃; under 6000480 Torr; for 72h; | 100% |
| With 14C2H2F3O(1-)*6C4H8O*La2Na8(14+) at 80℃; for 6h; Inert atmosphere; | 99% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
40195-27-5
(1-methoxy-2-phenylvinyloxy)trimethylsilane

| Conditions | Yield |
|---|---|
| With n-butyllithium; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 100% |
| Stage #1: benzeneacetic acid methyl ester With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 2.1h; Inert atmosphere; Stage #2: chloro-trimethyl-silane In tetrahydrofuran at 20℃; Inert atmosphere; | 100% |
| Stage #1: benzeneacetic acid methyl ester With lithium diisopropyl amide In tetrahydrofuran; hexane at -78℃; for 2h; Stage #2: chloro-trimethyl-silane In tetrahydrofuran; hexane at 20℃; | 91% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 35℃; under 6000480 Torr; for 72h; | 100% |
| With ammonium nitrate In neat (no solvent) at 20℃; for 12h; Reagent/catalyst; Green chemistry; | 99% |
| With magnesium chloride In tetrahydrofuran at 20℃; for 16h; | 98% |
| With potassium tert-butylate at 219℃; for 0.05h; microwave irradiation; | 63% |
| With bis(bis(trimethylsilyl)amido)tin(II) 1) hexane, r.t., 10 min; Yield given. Multistep reaction; |
-
-
110-89-4
piperidine

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
3626-62-8
2-phenyl-1-(1-piperidinyl)ethanone

| Conditions | Yield |
|---|---|
| In acetonitrile at 35℃; under 6000480 Torr; for 72h; | 100% |
| In acetonitrile at 45℃; under 6000480 Torr; for 72h; | 100% |
| With ammonium nitrate In neat (no solvent) at 20℃; for 12h; Green chemistry; | 99% |
-
-
75-30-9
2-iodo-propane

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
72615-27-1
2-phenyl-3-methylbutyric acid methyl ester

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium diisopropyl amide In tetrahydrofuran at -78℃; | 100% |
| With 2-pyrrolidinon In N,N-dimethyl-formamide Flow reactor; | 16% |
| With 1) EGB.2 1) -78 deg C, 2) -78 deg C, 15 min; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With tert-butylamine; lithium bromide for 6h; Heating; | 100% |
| With tetrachlorosilane for 8h; Heating; | 91% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; lithium bromide for 1h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With bis(tri-n-butyltin)oxide In toluene for 0.333333h; Product distribution; Irradiation; var. carboxylic ester, organotin oxide and hydroxide, solvent, time of temp. or microwave irrad.; | 100% |
| With bis(tri-n-butyltin)oxide In neat (no solvent) at 200℃; for 0.5h; | 100% |
| With aluminium trichloride; dodecyl methyl sulfide at 0 - 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; silica gel In hexane for 3h; Heating; | 100% |
| With methanol; sodium tetrahydroborate In diethyl ether at 20℃; for 38h; Reduction; | 96% |
| With sodium tetrahydroborate In diethylene glycol dimethyl ether at 104℃; | 95% |
-
-
101-41-7
benzeneacetic acid methyl ester

-
-
590-17-0
cyanomethyl bromide

-
-
88970-65-4
β-(carbomethoxy)-β-phenylpropionitrile

| Conditions | Yield |
|---|---|
| Stage #1: benzeneacetic acid methyl ester With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.333333h; Inert atmosphere; Stage #2: cyanomethyl bromide In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; | 100% |
| Stage #1: benzeneacetic acid methyl ester With n-butyllithium; 1,1,1,3,3,3-hexamethyl-disilazane In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: cyanomethyl bromide In tetrahydrofuran at -78 - 20℃; | 77% |
| Stage #1: benzeneacetic acid methyl ester With lithium diisopropyl amide In tetrahydrofuran; ethylbenzene; toluene at -78℃; for 1h; Inert atmosphere; Stage #2: cyanomethyl bromide In tetrahydrofuran; ethylbenzene; toluene at -78℃; Inert atmosphere; | 69% |
-
-
141-43-5
ethanolamine

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
6269-99-4
N-(2-hydroxyethyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With N,N'-Mes2imidazol-2-ylidene In tetrahydrofuran at 23℃; for 8h; | 100% |
| With 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,3,2-diazaphosphorine In acetonitrile at 20℃; for 15h; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 99% |
| With ammonium nitrate In neat (no solvent) at 20℃; for 12h; Green chemistry; | 99% |
-
-
27935-87-1
cyclopentylmethyl Iodide

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
933068-88-3
3-cyclopentyl-2-phenylpropionic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: benzeneacetic acid methyl ester With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.75h; Inert atmosphere; Stage #2: cyclopentylmethyl Iodide In tetrahydrofuran at -78 - 25℃; Inert atmosphere; | 100% |
-
-
111-42-2
2,2'-iminobis[ethanol]

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
7147-91-3
N,N-bis(2-hydroxyethyl)-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With ammonium nitrate In neat (no solvent) at 20℃; for 12h; Green chemistry; | 99% |
| at 100 - 150℃; |
-
-
107-11-9
1-amino-2-propene

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
30160-48-6
N-Allyl-2-phenylethanamide

| Conditions | Yield |
|---|---|
| With ammonium nitrate In neat (no solvent) at 20℃; for 12h; Green chemistry; | 99% |
-
-
67-63-0
isopropyl alcohol

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
4861-85-2
isopropyl phenylacetate

| Conditions | Yield |
|---|---|
| With tert-butylamine; lithium bromide for 33h; Heating; | 99% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; lithium bromide for 4h; Ambient temperature; | 94% |
| With indium; iodine for 6h; transesterification; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With C16H25N3O2S In n-heptane for 48h; Reflux; Molecular sieve; Inert atmosphere; | 99% |
| With 1,3-bis(3,5-bis(trifluoro-ethyl)phenyl)thiourea; 4-pyrrolidin-1-ylpyridine In octane for 6h; Reflux; | 98% |
| With SO3H and NH2+ functional carbon-based solid acid at 80℃; for 6h; | 92% |

| Conditions | Yield |
|---|---|
| With diisopropylamine; lithium diisopropyl amide In tetrahydrofuran; hexane -78 deg C, 1 h; -78 deg C to room temp., 35 min; | 99% |
-
-
124-40-3
dimethyl amine

-
-
101-41-7
benzeneacetic acid methyl ester

-
-
18925-69-4
N,N-dimethyl-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With magnesium chloride In tetrahydrofuran at 20℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With ammonium nitrate In neat (no solvent) at 20℃; for 12h; Green chemistry; | 99% |
| With potassium tert-butylate at 105℃; for 0.05h; microwave irradiation; | 97% |
| With trimethylaluminum In tetrahydrofuran; toluene at 125℃; for 0.0333333h; | 84% |
Methyl phenylacetate Consensus Reports
Methyl phenylacetate Specification
The Methyl benzeneacetate with CAS registry number of 101-41-7 is also called Benzeneaceticacid, methyl ester. The IUPAC name is methyl phenylacetate. Its EINECS registry number is 202-940-9. In addition, the molecular formula is C9H10O2 and the molecular weight is 150.17. It is a kind of colourless liquid and belongs to the classes of Organics; API Intermediates; Alphabetical Listings; Flavors and Fragrances; M-N; C8 to C9; Carbonyl Compounds; Esters. What's more, it is stable and incompatible with strong oxidizing agents and strong bases.
Physical properties about this chemical are: (1)ACD/LogP: 1.97; (2)ACD/LogD (pH 5.5): 1.97; (3)ACD/LogD (pH 7.4): 1.97; (4)ACD/BCF (pH 5.5): 18.37; (5)ACD/BCF (pH 7.4): 18.37; (6)ACD/KOC (pH 5.5): 279.56; (7)ACD/KOC (pH 7.4): 279.56; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.505; (12)Molar Refractivity: 42.2 cm3; (13)Molar Volume: 142.2 cm3; (14)Polarizability: 16.73 ×10-24cm3; (15)Surface Tension: 35.9 dyne/cm; (16)Density: 1.055 g/cm3; (17)Flash Point: 93.9 °C; (18)Enthalpy of Vaporization: 45.44 kJ/mol; (19)Boiling Point: 218 °C at 760 mmHg; (20)Vapour Pressure: 0.129 mmHg at 25°C.
Preparation of Methyl benzeneacetate: it can be prepared by benzene acetonitrile through hydrolysis esterification. This reaction will need reagent methanol and sulfuric acid. You should drop benzene acetonitrile in 1.5 hours at the temperature of 95 °C. The reaction time is 6 hours by heating at reaction temperature of 95-100 °C. Then go through the operation of colling, washing, discarding the water layer, fractionating to get the products. The yield is about 80%.
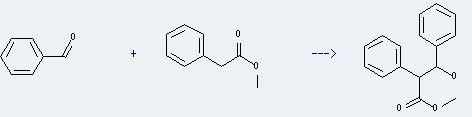
Uses of Methyl benzeneacetate: it can be used to modulate floral flavor. And it can be used for organic synthesis and used to manufacture atropine and anisodamine. In addition, it can react with benzaldehyde to Prepare 3-hydroxy-2,3-diphenyl-propionic acid methyl ester. This reaction will need reagent C6H5CHBrCO2CH3 and solvent dimethylformamide. The yield is about 35%.
When you are using this chemical, please be cautious about it as the following:
It is harmful if it contact with skin. During using it, do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer). In addition, you should avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC)Cc1ccccc1
(2)InChI: InChI=1/C9H10O2/c1-11-9(10)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3
(3)InChIKey: CRZQGDNQQAALAY-UHFFFAOYAC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 2400mg/kg (2400mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 941, 1974. | |
| rat | LD50 | oral | 2550mg/kg (2550mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 941, 1974. |
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2R)-2-bromo-2-(2-chlorophenyl)acetate
- Methyl (2R,3S)-3-(4-methoxyphenyl)-2-oxiranecarboxylate
- Methyl (2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate
- Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate
- Methyl (2S)-1-(1,2-dioxo-3,3-dimethypentyl)-2-pyrrolidinecarboxylate
- Methyl (2S)-2,3-epoxypropanoate
- Methyl (2S)-2-[(tert-butoxycarbonyl)amino]-3-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]propanoate
- Methyl (2S)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- 101418-00-2
- 101419-78-7
- 101420-79-5
- 101420-81-9
- 1014-23-9
- 1014-25-1
- 10142-59-3
- 101426-31-7
- 10142-78-6
- 101-42-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View