-
Name
Methyltriphenylphosphonium bromide
- EINECS 217-218-9
- CAS No. 1779-49-3
- Article Data45
- CAS DataBase
- Density
- Solubility 400 g/L in water at 25 °C
- Melting Point 230-234 °C(lit.)
- Formula C19H18P·Br
- Boiling Point
- Molecular Weight 357.23
- Flash Point >240°C
- Transport Information UN 1390 4.3/PG 2
- Appearance white powder
- Safety 36/37-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Methyltriphenylphosphonium bromide;Methyl(triphenyl)phosphonium bromide;Triphenylmethylphosphonium bromide;Phosphonium, methyltriphenyl-, bromide;
- PSA 13.59000
- LogP 0.61430
Synthetic route
-
-
1125-88-8
benzaldehyde dimethyl acetal

-
-
6399-81-1
triphenylphosphine hydrobromide

-
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide at 110℃; for 8h; | 99.2% |
| With hydrogen bromide at 110℃; for 15h; | 97% |
| With hydrogen bromide In water at 120℃; for 22.5h; | 97% |

| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 4h; | 99% |
| In benzene for 48h; Ambient temperature; | 95% |
| In acetonitrile |

| Conditions | Yield |
|---|---|
| In benzene | 99% |
-
-
6399-81-1
triphenylphosphine hydrobromide

-
-
149-73-5
trimethyl orthoformate

-
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| at 75℃; for 0.75h; Inert atmosphere; neat (no solvent); | 99% |
| at 70℃; | 70% |
-
-
1314805-21-4
methyl N-acetyl-α-triphenylphosphoniumglycinate bromide

-
A
-
1314805-28-1
(N-Acetylamino)methyltriphenylphosphonium bromide

-
B
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: methyl N-acetyl-α-triphenylphosphoniumglycinate bromide at 25℃; under 1 - 2 Torr; for 1h; Stage #2: With triphenylphosphine hydrobromide; triphenylphosphine at 100 - 115℃; for 1h; | A 98.6% B 87% |
-
-
603-35-0
triphenylphosphine

-
-
149-73-5
trimethyl orthoformate

-
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| With ammonium bromide at 110℃; for 4h; Sealed tube; | 96% |
| With ammonium bromide at 110℃; for 12h; | 96% |
| With cis,trans-24-bromotetracos-12-enal ethylene acetal |

-
A
-
498572-96-6
C20H18N2O6

-
B
-
1779-49-3
Methyltriphenylphosphonium bromide

-
C
-
28387-29-3
α-(N-benzoylamino)methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: methyl N-benzoyl-α-triphenylphosphonioglycinate bromide at 25℃; under 1 - 2 Torr; for 1h; Stage #2: With triphenylphosphine hydrobromide; triphenylphosphine at 110 - 120℃; for 1h; | A 6.2% B 81% C 89.1% |

-
-
1314805-22-5
Br(1-)*C26H29NO3P(1+)

-
A
-
1314805-30-5
Br(1-)*C24H27NOP(1+)

-
B
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: Br(1-)*C26H29NO3P(1+) at 25℃; under 1 - 2 Torr; for 1h; Stage #2: With triphenylphosphine hydrobromide; triphenylphosphine at 120℃; for 1.5h; | A 78.7% B 44.5% |
-
-
19320-74-2
Se-phenacyldimethylselenonium bromide

-
-
603-35-0
triphenylphosphine

-
A
-
3878-44-2
Triphenylphosphine selenide

-
B
-
19859-29-1
2-(methylselanyl)-1-phenylethan-1-one

-
C
-
1779-49-3
Methyltriphenylphosphonium bromide

-
D
-
6048-29-9
triphenylphenacylphosphonium bromide

| Conditions | Yield |
|---|---|
| In benzene for 6h; Heating; Further byproducts given; | A 7.3% B n/a C 13% D 73% |

-
-
19320-74-2
Se-phenacyldimethylselenonium bromide

-
-
603-35-0
triphenylphosphine

-
A
-
3878-44-2
Triphenylphosphine selenide

-
B
-
19859-29-1
2-(methylselanyl)-1-phenylethan-1-one

-
C
-
1779-49-3
Methyltriphenylphosphonium bromide

-
D
-
6048-29-9
triphenylphenacylphosphonium bromide

-
E
-
98-86-2
acetophenone

| Conditions | Yield |
|---|---|
| In benzene for 6h; Product distribution; Mechanism; Heating; | A 7.3% B n/a C 13% D 73% E n/a |


-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
B
-
28387-29-3
α-(N-benzoylamino)methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| With triphenylphosphine hydrobromide; triphenylphosphine at 115℃; under 1 - 2 Torr; for 1.5h; | A n/a B 71.5% |
| Stage #1: methyl N-benzoyl-α-triphenylphosphonioglycinate bromide at 25℃; under 1 - 2 Torr; for 1h; Stage #2: at 155℃; for 0.5h; | A 55.4% B 27% |

| Conditions | Yield |
|---|---|
| With oct-1-ene; nickel dibromide In tetrahydrofuran Heating; | 56% |
-
-
1314805-21-4
methyl N-acetyl-α-triphenylphosphoniumglycinate bromide

-
B
-
1314805-28-1
(N-Acetylamino)methyltriphenylphosphonium bromide

-
C
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: methyl N-acetyl-α-triphenylphosphoniumglycinate bromide at 25℃; under 1 - 2 Torr; for 1h; Stage #2: at 140 - 150℃; for 0.5h; | A 19.4% B 49.5% C 37.2% |

-
-
19493-09-5
Methylenetriphenylphosphorane

-
A
-
111441-95-3
(chloromethyl)triphenylphosphonium bromide

-
B
-
1779-49-3
Methyltriphenylphosphonium bromide

-
C
-
1826-86-4
Dichlormethyl-triphenyl-phosphonium-bromid

| Conditions | Yield |
|---|---|
| With 1,1-difluorotetrachloroethane In diethyl ether at -78℃; for 0.5h; | A 40% B 35% C 15% |

-
-
19493-09-5
Methylenetriphenylphosphorane

-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
B
-
98822-67-4
iodomethyl triphenylphosphonium bromide

-
C
-
117747-27-0
Diiodomethyl-triphenyl-phosphonium; bromide

| Conditions | Yield |
|---|---|
| With 1-chloro-1,1,2,2-tetrafluoro-2-iodo-ethane In diethyl ether at -78℃; for 0.5h; | A 31% B 40% C 5 % Spectr. |

-
-
1314805-22-5
Br(1-)*C26H29NO3P(1+)

-
A
-
498572-95-5
C16H26N2O6

-
B
-
1314805-30-5
Br(1-)*C24H27NOP(1+)

-
C
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| Stage #1: Br(1-)*C26H29NO3P(1+) at 25℃; under 1 - 2 Torr; for 1h; Stage #2: at 120℃; for 0.5h; | A 22.9% B 34.5% C 21.8% |

-
-
19493-09-5
Methylenetriphenylphosphorane

-
A
-
1034-49-7
(bromomethyl)triphenyl phosphonium bromide

-
B
-
1779-49-3
Methyltriphenylphosphonium bromide

-
C
-
56506-89-9
tribromomethyltriphenylphosphonium bromide

-
D
-
56506-90-2
(dibromomethyl)(triphenyl)phosphonium bromide

| Conditions | Yield |
|---|---|
| With 1,2-dibromo-1,1,2,2-tetrafluoroethane In diethyl ether at -78℃; for 0.5h; | A 18% B 35 % Spectr. C 24 % Spectr. D 13 % Spectr. |

-
-
100074-67-7
5-bromo-4-methoxy-3-phenylfuran-2(5H)-one

-
-
603-35-0
triphenylphosphine

-
A
-
74-83-9
methyl bromide

-
B
-
1779-49-3
Methyltriphenylphosphonium bromide

-
C
-
100074-79-1
2,5-dihydro-2-oxo-3-phenyl-5-triphenylphosphoniumfuran-4-olate

| Conditions | Yield |
|---|---|
| In benzene for 2h; Product distribution; Mechanism; Heating; | A n/a B 8% C 65.3 g |
| In benzene for 2h; Heating; | A n/a B 8% C 65.3 g |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| In methanol |
-
-
6399-81-1
triphenylphosphine hydrobromide

-
-
100-66-3
methoxybenzene

-
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| at 160 - 170℃; |
-
-
89940-00-1
4-bromo-1,1-dimethoxypentane

-
-
603-35-0
triphenylphosphine

-
-
1779-49-3
Methyltriphenylphosphonium bromide
-
-
558-30-5
2-methyl-1,2-epoxypropane

-
-
603-35-0
triphenylphosphine

-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
B
-
53101-88-5
(2-Methyl-propenyl)-triphenyl-phosphonium; bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; phenol 1.) 100 deg C, 24 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
98560-25-9
epoxy-1,2 propyl-2 pentane

-
-
603-35-0
triphenylphosphine

-
-
1779-49-3
Methyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; phenol 1.) 100 deg C, 24 h; Yield given. Multistep reaction; |
-
-
84451-53-6
cis-24-bromotetracos-12-enal ethylene acetal

-
-
603-35-0
triphenylphosphine

-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
B
-
113309-15-2
cis-23-(dioxolan-2-yl)tricos-12-enyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| With trimethyl orthoformate |

-
-
113309-14-1
cis-24-bromotetracos-12-enal ethylene acetal

-
-
603-35-0
triphenylphosphine

-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
B
-
113309-16-3
trans-23-(dioxolan-2-yl)tricos-12-enyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| With trimethyl orthoformate |

-
-
603-35-0
triphenylphosphine

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
B
-
3020-30-2
(2-hydroxypropyl)triphenylphosphonium bromide

-
C
-
28691-76-1
(E)-prop-1-en-1-yl-triphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| Product distribution; multistep reaction; variation of temperature and reaction time; | |
| With hydrogen bromide; phenol 1.) 100 deg C, 24 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
603-35-0
triphenylphosphine

-
-
2984-50-1
1,2-Epoxyoctane

-
A
-
1779-49-3
Methyltriphenylphosphonium bromide

-
C
-
56318-76-4, 88518-02-9
((E)-Oct-2-enyl)-triphenyl-phosphonium; bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; phenol 1.) 100 deg C, 24 h; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran Stage #2: benzophenone In tetrahydrofuran at 20℃; for 6h; Wittig reaction; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Stage #2: benzophenone In tetrahydrofuran at 20℃; Inert atmosphere; | 94% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In diethyl ether at 20℃; for 0.25h; Inert atmosphere; Stage #2: benzophenone In diethyl ether at 0℃; for 15h; Inert atmosphere; | 87% |
-
-
19099-93-5
benzyl 4-oxo-1-piperidinecarboxylate

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
138163-12-9
benzyl 4-methylene-1-piperidinecarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 1h; Stage #2: benzyl 4-oxo-1-piperidinecarboxylate In tetrahydrofuran at 0 - 20℃; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran at 0℃; for 2h; Wittig Reaction; Inert atmosphere; Stage #2: benzyl 4-oxo-1-piperidinecarboxylate In tetrahydrofuran at 0 - 20℃; for 4h; Wittig Reaction; Inert atmosphere; | 92% |
| Stage #1: Methyltriphenylphosphonium bromide With lithium hexamethyldisilazane In tetrahydrofuran at 0℃; for 1h; Stage #2: benzyl 4-oxo-1-piperidinecarboxylate In tetrahydrofuran for 2h; | 81% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
91547-83-0
(1R,4aS,8aS)-(-)-1,2,3,4,4a,5,6,7,8,8a-decahydro-5,5,8a-trimethyl-2-oxo-1-naphthaleneacetonitrile

-
-
124462-80-2
(1S,4aS,8aS)-(+)-1,2,3,4,4a,5,6,7,8,8a-decahydro-5,5,8a-trimethyl-2-methylene-1-naphthaleneacetonitrile

| Conditions | Yield |
|---|---|
| With sodium amide In tetrahydrofuran at 20℃; for 3.5h; Wittig reaction; | 100% |
| With n-butyllithium 1. DME, n-hexane, 0 deg C, 1.5 h 2. DME, 2 h, room temperature; Yield given. Multistep reaction; | |
| With sodium hydride 1.) DMSO, 15 min, 2.) DMSO, 75 deg C, 5 h; Yield given. Multistep reaction; |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
19493-09-5
Methylenetriphenylphosphorane

| Conditions | Yield |
|---|---|
| With ammonia; sodium amide | 100% |
| With sodium hexamethyldisilazane In diethyl ether Glovebox; Inert atmosphere; | 98% |
| With phenyllithium In dibutyl ether at 0 - 20℃; for 3.5h; Inert atmosphere; Glovebox; Schlenk technique; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With water for 0.5h; Reflux; Stage #2: With sodium hydroxide for 2h; Reflux; | 100% |
| With sodium hydroxide for 1h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20℃; for 2h; Wittig Olefination; Stage #2: salicylaldehyde In tetrahydrofuran at -78 - 30℃; Wittig Olefination; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20℃; for 2h; Stage #2: salicylaldehyde In tetrahydrofuran at -78 - 20℃; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 23℃; for 2h; Stage #2: salicylaldehyde In tetrahydrofuran at -78 - 23℃; for 20h; | 98% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
6630-33-7
ortho-bromobenzaldehyde

-
-
2039-88-5
2-bromostyrene

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20℃; for 2h; Wittig Olefination; Stage #2: ortho-bromobenzaldehyde In tetrahydrofuran at 0 - 20℃; for 12h; Wittig Olefination; | 100% |
| With n-butyllithium In tetrahydrofuran | 99% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In diethyl ether at 20℃; for 0.25h; Inert atmosphere; Stage #2: ortho-bromobenzaldehyde In diethyl ether at 0℃; for 15h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In toluene at 0 - 20℃; for 4.5h; Stage #2: 1-naphthaldehyde In toluene at 0 - 20℃; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In toluene at 0 - 20℃; for 4.5h; Stage #2: 1-naphthaldehyde In toluene at 0 - 20℃; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In toluene at 0 - 20℃; for 4.5h; Stage #2: 1-naphthaldehyde In toluene at 0 - 20℃; | 100% |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
16939-57-4
(E)-1-Phenyl-1,3-butadiene

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.25h; Inert atmosphere; Stage #2: (E)-3-phenylpropenal In tetrahydrofuran; hexane at 0 - 20℃; Inert atmosphere; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; cyclohexane at 0℃; for 0.25h; Stage #2: (E)-3-phenylpropenal In tetrahydrofuran; cyclohexane at 0 - 20℃; for 2h; Wittig olefination; | 96% |
| Stage #1: Methyltriphenylphosphonium bromide In tetrahydrofuran; hexane at 0℃; for 0.25h; Inert atmosphere; Stage #2: (E)-3-phenylpropenal In tetrahydrofuran; hexane at 0 - 20℃; for 10h; Inert atmosphere; | 96% |
-
-
4746-97-8
cyclohexanedione monoethylene ketal

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
51656-90-7
8-methylene-1,4-dioxaspiro[4.5]decane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In benzene at 25℃; for 1h; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at -78 - 0℃; for 0.666667h; Stage #2: cyclohexanedione monoethylene ketal In tetrahydrofuran; hexane at 20℃; | 92% |
| With sodium hydride In dimethyl sulfoxide | 90% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
114077-82-6
4-chloronicotinaldehyde

-
-
223573-95-3
4-chloro-3-vinylpyridine

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran | 100% |
| With tert.-butyl lithium In diethyl ether; pentane at -78 - 20℃; Wittig Olefination; Inert atmosphere; | 50% |
| With n-butyllithium In tetrahydrofuran |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
289666-94-0
(+)-(3S,4R,5R,6R)-5-benzyloxy-7-tert-butyldiphenylsilyloxy-2,2-dimethyl-3-methoxy-4,6-methylenedioxyheptanal

-
-
289667-02-3
(+)-(4S,5R,6R,7R)-6-benzyloxy-8-tert-butyldiphenylsilyloxy-3,3-dimethyl-4-methoxy-5,7-methylenedioxyoctyl-1-ene

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 65℃; for 0.25h; Stage #2: (+)-(3S,4R,5R,6R)-5-benzyloxy-7-tert-butyldiphenylsilyloxy-2,2-dimethyl-3-methoxy-4,6-methylenedioxyheptanal In tetrahydrofuran; hexane at 0℃; for 0.333333h; Wittig reaction; | 100% |
| With n-butyllithium at 0 - 20℃; Wittig reaction; | 100% |
-
-
288156-11-6
4,15-diformyl[2.2]paracyclophane

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
111870-60-1
pseudo-gem-divinyl<2.2>paracyclophane

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; Wittig reaction; Inert atmosphere; Stage #2: 4,15-diformyl[2.2]paracyclophane In tetrahydrofuran Wittig reaction; Inert atmosphere; Cooling with ice; | 100% |
| With n-butyllithium In tetrahydrofuran; hexane at 20℃; for 18h; Wittig reaction; | 85% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
307949-94-6
1-O-Benzyl-2-O-[(3R,7R,11R)-3,7,11,15-tetramethylhexadecanyl]-3-O-[(3R,7R,11S,15S)-15-formyl-3,7,11,15-tetramethylpentadecanyl]-sn-glycerol

-
-
307949-95-7
1-O-Benzyl-2-O-[(3R,7R,11R)-3,7,11,15-tetramethylhexadecanyl]-3-O-[(3R,7R,11S,15S)-3,7,11,15-tetramethylheptadec-16-enyl]-sn-glycerol

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In hexane at -78 - 20℃; for 0.666667h; Dehydrobromination; Stage #2: 1-O-Benzyl-2-O-[(3R,7R,11R)-3,7,11,15-tetramethylhexadecanyl]-3-O-[(3R,7R,11S,15S)-15-formyl-3,7,11,15-tetramethylpentadecanyl]-sn-glycerol In tetrahydrofuran; hexane at -78 - -25℃; for 1.25h; Condensation; Wittig reaction; Further stages.; | 100% |
-
-
34041-01-5
4-isopropyl-5-oxo-hexanenitrile

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
365978-53-6
4-isopropyl-5-methyl-hex-5-enenitrile

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With sodium hydride In dimethyl sulfoxide at 25℃; Stage #2: 4-isopropyl-5-oxo-hexanenitrile In dimethyl sulfoxide for 1h; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
383196-60-9
(S)-4-Allyl-1,5,5-trimethyl-cyclopentane-1,2-diol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 0.5h; Wittig olefination; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
310398-06-2
(6R)-(t-butyldimethylsilanyloxymethyl)-(2R)-ethynyl-(3R)-(triisopropylsilanyloxy)-tetrahydropyran-4-one

-
-
310398-07-3
(6R)-(t-butyldimethylsilanyloxymethyl)-(2R)-ethynyl-4-methylene-(3S)-(triisopropylsilanoxy)-tetrahydropyran

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 20℃; for 1.5h; Stage #2: (6R)-(t-butyldimethylsilanyloxymethyl)-(2R)-ethynyl-(3R)-(triisopropylsilanyloxy)-tetrahydropyran-4-one In tetrahydrofuran; hexane at 20℃; for 2.5h; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide for 72h; | 100% |
-
-
19005-93-7
1H-indol-2-ylcarboxaldehyde

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
53654-35-6
2-vinyl-1H-indole

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium hexamethylsilazane In tetrahydrofuran; toluene at 20℃; for 0.5h; Stage #2: 1H-indol-2-ylcarboxaldehyde In tetrahydrofuran; toluene at 20℃; for 2h; Wittig reaction; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With sodium hexamethyldisilazane In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 1H-indol-2-ylcarboxaldehyde In tetrahydrofuran at 20℃; for 14h; Inert atmosphere; | 92% |
| With potassium hexamethylsilazane In tetrahydrofuran; toluene at 23℃; for 3h; | 90% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
478942-83-5
2-isopropoxybiphenyl-3-carbaldehyde

-
-
478942-85-7
2-isopropoxy-3-vinyl-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 22℃; for 2h; Stage #2: 2-isopropoxybiphenyl-3-carbaldehyde In tetrahydrofuran at -78 - 22℃; Wittig olefination; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With sodium t-butanolate In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 2-isopropoxybiphenyl-3-carbaldehyde In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 99% |
| With potassium tert-butylate In diethyl ether at 0℃; Wittig olefination; | 88% |
| Stage #1: Methyltriphenylphosphonium bromide With sodium t-butanolate In diethyl ether at 20℃; for 0.5h; Wittig Olefination; Inert atmosphere; Stage #2: 2-isopropoxybiphenyl-3-carbaldehyde In diethyl ether at 20℃; for 0.5h; Wittig Olefination; Inert atmosphere; | 55% |
| With potassium tert-butylate In diethyl ether at 0℃; for 0.166667h; Wittig reaction; |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
519033-40-0
3-formyl-1H-indole-2-carboxylic acid (but-3-enyl)amide

-
-
519033-41-1
3-vinyl-1H-indole-2-carboxylic acid (but-3-enyl)amide

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0 - 20℃; for 3h; Stage #2: 3-formyl-1H-indole-2-carboxylic acid (but-3-enyl)amide In tetrahydrofuran; hexane at 20℃; for 2h; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
481656-25-1
(1S,4aS,8aR)-1α-(2-methoxybenzyl)-1β,2β,4aβ-trimethyl-1,2,3,4,4a,7,8,8aα-octahydronaphthalene-5(6H)-one

-
-
481656-15-9
(1S,4aS,8aR)-1α-(2-methoxybenzyl)-1β,2β,4aβ-trimethyl-5-(methylene)decahydronaphthalene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In benzene Heating; | 100% |
| With potassium tert-butylate In benzene for 12h; Wittig reaction; Heating; | 100% |
| With potassium tert-butylate In benzene for 12h; Wittig reaction; heating; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In benzene for 2h; Wittig methylenation; Inert atmosphere; Reflux; Stage #2: (1S,4aS,8aR)-1α-(2-methoxybenzyl)-1β,2β,4aβ-trimethyl-1,2,3,4,4a,7,8,8aα-octahydronaphthalene-5(6H)-one In benzene for 12h; Wittig methylenation; Inert atmosphere; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide; C91H108O17 With sodium hexamethyldisilazane In tetrahydrofuran at 0℃; for 1h; Wittig reaction; Stage #2: Methyltriphenylphosphonium bromide With sodium hexamethyldisilazane In tetrahydrofuran at 0℃; for 0.5h; Wittig reaction; Further stages.; | 100% |
| Stage #1: Methyltriphenylphosphonium bromide With sodium hexamethyldisilazane In tetrahydrofuran at 0℃; for 0.333333h; Stage #2: C91H108O17 In tetrahydrofuran at 0℃; for 0.5h; |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
107811-48-3
2-isopropoxy-4-methoxybenzene-1-carboaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 22℃; for 2h; Stage #2: 2-isopropoxy-4-methoxybenzene-1-carboaldehyde In tetrahydrofuran at -78 - 22℃; Wittig olefination; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
623902-72-7
2-isopropoxy-5-methoxy-1-vinylbenzene

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 22℃; for 2h; Stage #2: 2-isopropoxy-5-methoxybenzaldehyde In tetrahydrofuran at -78 - 22℃; Wittig olefination; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran Wittig reaction; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
654680-31-6
(1R,5S,6S)-5-benzyloxymethyl-6-(2-propenyl)-2-cyclohex-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With sodium hexamethyldisilazane In tetrahydrofuran for 0.25h; Stage #2: (3aS,4S,7aR)-4-Benzyloxymethyl-2,3,3a,4,5,7a-hexahydro-benzofuran-2-ol In tetrahydrofuran at -78℃; for 0.333333h; Wittig reaction; Stage #3: In tetrahydrofuran for 2h; Wittig reaction; Heating; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
749924-32-1
(E)-3-[(Z)-(2R,8S,9R)-8-(tert-Butyl-diphenyl-silanyloxy)-9-(tert-butyl-diphenyl-silanyloxymethyl)-2,3,4,7,8,9-hexahydro-oxonin-2-yl]-2-methyl-propenal

-
-
749924-33-2
(Z)-(2R,3S,9R)-3-(tert-Butyl-diphenyl-silanyloxy)-2-(tert-butyl-diphenyl-silanyloxymethyl)-9-((E)-2-methyl-buta-1,3-dienyl)-2,3,4,7,8,9-hexahydro-oxonine

| Conditions | Yield |
|---|---|
| With n-butyllithium at -78℃; for 2h; Wittig methylenation; | 100% |
-
-
31477-10-8
2,2'-diformyl-5,5',6,6'-tetramethoxybiphenyl

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
790220-94-9
2,2'-divinyl-5,5',6,6'-tetramethoxybiphenyl

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at 20℃; for 24h; Wittig reaction; | 100% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
247191-04-4
(2R,3R,4S,5R)-2,3-O-isopropylidene-2,3-dihydroxy-4,5-epoxycyclopentanone

-
-
247191-05-5
(2S,3S,4S,5R)-1-methylene-2,3-O-isopropylidene-2,3-dihydroxy-4,5-epoxycyclopentane

| Conditions | Yield |
|---|---|
| With sodium amide In tetrahydrofuran at 25℃; for 1h; | 100% |
Methyl triphenyl phosphonium bromide Specification
The Phosphonium,methyltriphenyl-, bromide (1:1), with the CAS registry number 1779-49-3, is also known as Triphenylmethylphosphonium bromide. It belongs to the product categories of Phosphonium Compounds; Synthetic Organic Chemistry; Wittig & Horner-Emmons Reaction; Wittig Reaction; C-C Bond Formation; Olefination; Wittig Reagents; Organophosphorus compound. Its EINECS number is 217-218-9. This chemical's molecular formula is C19H18P·Br and molecular weight is 357.22. What's more, its systematic name is Methyl(triphenyl)phosphonium bromide. This chemical is stable at common pressure and temperature, and it should be sealed and stored in a cool and dry place. Moreover, it should be protected from heat and fire.
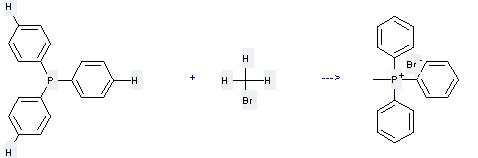
Preparation of Phosphonium,methyltriphenyl-, bromide (1:1): this chemical can be prepared by triphenylphosphane and bromomethane at the ambient temperature. This reaction will need solvent benzene with the reaction time of 2 days. The yield is about 95%.
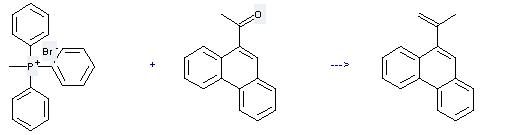
Uses of Phosphonium,methyltriphenyl-, bromide (1:1): it can be used to produce 9-isopropenyl-phenanthrene. It will need reagent NaNH2 and solvent tetrahydrofuran. The yield is about 94%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin and can cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing and gloves.
You can still convert the following datas into molecular structure:
(1)SMILES: [Br-].c1(ccccc1)[P+](c2ccccc2)(c3ccccc3)C
(2)Std. InChI: InChI=1S/C19H18P.BrH/c1-20(17-11-5-2-6-12-17,18-13-7-3-8-14-18)19-15-9-4-10-16-19;/h2-16H,1H3;1H/q+1;/p-1
(3)Std. InChIKey: LSEFCHWGJNHZNT-UHFFFAOYSA-M
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 1779-51-7
- 17795-21-0
- 177952-38-4
- 177952-39-5
- 1779-54-0
- 1779-58-4
- 177966-52-8
- 177966-58-4
- 177966-59-5
- 177966-60-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View