-
Name
N,N-Dimethylbenzamide
- EINECS 210-279-2
- CAS No. 611-74-5
- Article Data274
- CAS DataBase
- Density 1.03 g/cm3
- Solubility Soluble in water.
- Melting Point 41-45 °C
- Formula C9H11NO
- Boiling Point 256.1 °C at 760 mmHg
- Molecular Weight 149.192
- Flash Point 111.9 °C
- Transport Information
- Appearance white to off-white crystals.
- Safety 26-36-37/39
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms Dimethylbenzamide;NSC 10996;
- PSA 20.31000
- LogP 1.38840
Synthetic route
-
-
93-58-3
benzoic acid methyl ester

-
-
84738-97-6
methylchloroaluminum N,N-dimethylamide

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| In benzene for 12h; Heating; | 100% |
-
-
15482-60-7
N,N-dimethylthiobenzamide

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With 6H(1+)*Mo9O40PV3(6-); oxygen In acetonitrile at 90℃; under 760.051 Torr; for 2h; Inert atmosphere; Glovebox; | 99% |
| With hydrogenchloride; N-nitrosopiperidine; potassium iodide In dichloromethane; water at 22℃; for 50h; | 94% |
| With hydrogenchloride; sodium nitrite In water at 20℃; for 0.5h; | 94% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In acetonitrile at 80℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; copper(II) oxide In water at 80℃; for 20h; Catalytic behavior; | 99% |
| With tert.-butylhydroperoxide; copper(II) perchlorate hexahydrate at 100℃; for 15h; | 90% |
| With trichlorophosphate at 120℃; for 1h; Sealed tube; | 90% |

| Conditions | Yield |
|---|---|
| In 5,5-dimethyl-1,3-cyclohexadiene for 12h; Reflux; | 99% |
-
-
98-88-4
benzoyl chloride

-
A
-
2930-27-0
2,4,6-Triphenyl-thiopyrylium-chlorid

-
B
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With 2-Dimethylamino-2,4,6-triphenyl-2H-thiopyran In diethyl ether for 1h; Heating; | A 98% B 40% |
-
-
87691-75-6
2-Dimethylamino-2,4,6-triphenyl-2H-thiopyran

-
A
-
2930-27-0
2,4,6-Triphenyl-thiopyrylium-chlorid

-
B
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With benzoyl chloride In diethyl ether for 1h; Heating; | A 98% B 40% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane at 0℃; for 0.5h; | 98% |
| With tetrabutylammomium bromide; iodine; potassium hydroxide In water at 20℃; for 2.5h; Irradiation; | 75% |
| With dichloro(dimethylglyoxime)(dimethylglyoximato)cobalt(III); [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate; triphenylphosphine In dichloromethane at 20℃; for 12h; Inert atmosphere; Sealed tube; Irradiation; | 74% |
| Stage #1: dimethyl amine; benzoic acid at 60℃; Autoclave; Cooling with dry ice; Stage #2: at 290℃; under 22502.3 Torr; for 0.0136111h; Microwave irradiation; |

| Conditions | Yield |
|---|---|
| With sodium methylate; nickel diacetate In 1,4-dioxane at 110℃; | 98% |
| Stage #1: N,N-dimethyl-formamide With 1,3,5-trichloro-2,4,6-triazine at 20℃; for 1h; Stage #2: bromobenzene With 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate In N,N-dimethyl-formamide at 120℃; for 6h; | 81% |
| With palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; trichlorophosphate at 165℃; for 24h; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| With Pd nanoparticles supported on reduced graphene oxide nanosheets at 200℃; for 6h; Temperature; Concentration; Autoclave; | 98% |
| With palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; trichlorophosphate at 135℃; for 6h; Inert atmosphere; | 93% |
| Stage #1: N,N-dimethyl-formamide With tungsten(VI) chloride at 20℃; for 0.25h; Inert atmosphere; Stage #2: iodobenzene With palladium dichloride at 140℃; for 5h; Catalytic behavior; Reagent/catalyst; Temperature; Inert atmosphere; | 93% |
-
-
35452-04-1
N,N-dimethylbenzamide dimethyl acetal

-
B
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With trichloroacetamidine In tetrahydrofuran at 40 - 50℃; for 3h; Title compound not separated from byproducts; | A 96% B 5% |
-
-
35452-04-1
N,N-dimethylbenzamide dimethyl acetal

-
-
2533-68-8
trichloroacetamidine

-
B
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 40 - 50℃; for 3h; Title compound not separated from byproducts; | A 96% B 5% |

-
-
101137-69-3
tert-butyl benzoyl(phenyl)carbamate

-
-
124-40-3
dimethyl amine

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 23℃; for 15h; Inert atmosphere; Schlenk technique; | 96% |
-
-
120-46-7
1,3-diphenylpropanedione

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With iodosylbenzene at 25℃; for 24h; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; Inert atmosphere; | 95% |
| With triethylamine In dichloromethane at 0 - 20℃; | 93% |
| With dmap; triethylamine In tetrahydrofuran; dichloromethane at 0 - 20℃; for 12h; | 91% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 23℃; for 15h; Inert atmosphere; Schlenk technique; | 95% |
-
-
74542-54-4
N,N-phenyl-p-toluenesulfonylbenzamide

-
-
124-40-3
dimethyl amine

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 23℃; for 15h; Inert atmosphere; Schlenk technique; | 94% |
-
-
54616-47-6
2-bromo-N,N-dimethyl benzamide

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; potassium tert-butylate; isopropyl alcohol at 100℃; Schlenk technique; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| In chloroform for 1h; | 92% |

| Conditions | Yield |
|---|---|
| With sodium iodide In dimethyl sulfoxide at 20℃; for 6h; Solvent; Reagent/catalyst; Electrochemical reaction; | 92% |

| Conditions | Yield |
|---|---|
| With dysprosium at 20℃; for 0.333333h; Inert atmosphere; | 91% |
| Stage #1: N,N-dimethyl-formamide With potassium tert-butylate In tetrahydrofuran at 20℃; for 0.25h; Schlenk technique; Stage #2: benzoyl chloride In tetrahydrofuran at 50℃; for 12h; | 84% |
-
-
98-88-4
benzoyl chloride

-
-
79962-35-9
2-Dimethylamino-3-methyl-2,4,6-triphenyl-2H-pyran

-
A
-
92919-44-3
3-Methyl-2,4,6-triphenylpyryliumchlorid

-
B
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| In diethyl ether for 1h; Heating; | A 91% B 54% |

-
-
79962-35-9
2-Dimethylamino-3-methyl-2,4,6-triphenyl-2H-pyran

-
A
-
92919-44-3
3-Methyl-2,4,6-triphenylpyryliumchlorid

-
B
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With benzoyl chloride In diethyl ether for 1h; Heating; | A 91% B 54% |

| Conditions | Yield |
|---|---|
| With oxygen; sodium hydroxide In water at 25℃; for 24h; Green chemistry; | 91% |
| With oxygen; sodium hydroxide In water at 25℃; for 24h; | 90% |
| With tert.-butylhydroperoxide; [bis(acetoxy)iodo]benzene In acetonitrile Reagent/catalyst; Solvent; Reflux; | 86% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine at 70 - 80℃; for 24h; | 91% |
| With hydrogenchloride; tert.-butylhydroperoxide; iodine In cyclohexane; water at 80℃; | 88% |
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide at 80℃; for 24h; | 85% |

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With (R)-(+)-2,2'-bis[bis(3,5-dimethylphenyl)phosphino]-1,1'-binaphthyl; bis(1,5-cyclooctadiene)iridium(I) tetrakis[3,5-bis(trifluoromethyl)phenyl]borate In tetrahydrofuran at 135℃; for 24h; Reagent/catalyst; Sealed tube; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| bis(di-tert-butylphosphinous acid)PdCl2 In acetonitrile at 25℃; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; UV-irradiation; | 90% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 88% |

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| In n-heptane soln. was refluxed for 5 h, after cooling, heptane was removed under vac.; elem.anal.; | 100% |

| Conditions | Yield |
|---|---|
| With C37H46FeN4; diphenylsilane In tetrahydrofuran at 70℃; for 24h; Schlenk technique; Inert atmosphere; | 99% |
| With Pt(ItBu)(divinyltetramethyldisiloxane); diphenylsilane In tetrahydrofuran; Hexadecane at 40℃; for 1h; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; | 99% |
| With triethyl borane; phenylsilane; sodium hydroxide In tetrahydrofuran; tert-butyl methyl ether at 20℃; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 99% |
-
-
611-74-5
N,N-dimethylbenzamide

-
-
15482-60-7
N,N-dimethylthiobenzamide

| Conditions | Yield |
|---|---|
| With thiophosphorylated amine resin In toluene at 90℃; for 30h; | 99% |
| With tetraphosphorus decasulfide In dichloromethane for 0.25h; Inert atmosphere; Reflux; | 99% |
| With tetraphosphorus decasulfide; sodium carbonate In tetrahydrofuran at 25℃; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; copper diacetate; C46H44I4Ir2N2O4 In 1,4-dioxane at 100℃; for 24h; Glovebox; Inert atmosphere; | 99% |
-
-
128-08-5
N-Bromosuccinimide

-
-
611-74-5
N,N-dimethylbenzamide

-
-
87329-69-9
N-methyl-N-succinimidomethylbenzamide

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In tetrachloromethane Product distribution; Heating; succinimidation by N-bromosuccinimide of N,N-dimethylamides and N,N-dimethylamines; | 98% |
| With dibenzoyl peroxide In tetrachloromethane Heating; | 98% |

| Conditions | Yield |
|---|---|
| With n-butyllithium; borane-THF; diisopropylamine at 25℃; | 98% |
| With diisobutylaluminum borohydride In tetrahydrofuran at 0 - 25℃; Inert atmosphere; | 98% |
| With diisobutylaluminum borohydride In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; | 98% |
-
-
141-32-2
acrylic acid n-butyl ester

-
-
611-74-5
N,N-dimethylbenzamide

-
-
1373338-22-7
N,N-dimethyl-2-[(E)-(n-butoxycarbonyl)ethenyl]benzamide

| Conditions | Yield |
|---|---|
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; Ag(1+)*(x)F6Sb; copper(II) acetate monohydrate In tert-Amyl alcohol at 100℃; for 4h; Inert atmosphere; regioselective reaction; | 98% |
| With silver hexafluoroantimonate; tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer In chlorobenzene at 80℃; for 16h; Heck Reaction; Irradiation; | 91% |

| Conditions | Yield |
|---|---|
| With lithium diisobutylmorpholinoaluminum hydride In tetrahydrofuran; hexane at 0℃; for 0.5h; Concentration; Time; | 97% |
| Stage #1: N,N-dimethylbenzamide With chloromagnesium dimethylaminoborohydride In tetrahydrofuran at 25℃; for 0.5h; Inert atmosphere; Stage #2: With acetaldehyde; acetic acid In tetrahydrofuran; pentane for 5h; Inert atmosphere; | 74% |
| With diethyl ether; diisobutylaluminium hydride |

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 30 - 40℃; Microwave irradiation; | 97% |
-
-
55-81-2
4-Methoxyphenethylamine

-
-
611-74-5
N,N-dimethylbenzamide

-
-
3278-19-1
N-[2-(4-methoxyphenyl)ethyl]benzamide

| Conditions | Yield |
|---|---|
| With Imidazole hydrochloride at 150℃; for 3h; Sealed tube; | 97% |
-
-
59983-62-9
Li{(CH2)(CH2)P(C6H5)2}

-
-
611-74-5
N,N-dimethylbenzamide

-
A
-
19023-66-6
Methyl-diphenyl-phenacyl-phosphonium

-
B
-
98-86-2
acetophenone

-
C
-
2129-89-7
diphenyl(methyl)phosphine oxide

| Conditions | Yield |
|---|---|
| With HX In water 1.) 2 h, -55 deg C; 2.) 13 h, 21 deg C; | A 96% B 20% C 20% |
| With HX In water 1.) 2 h, -55 deg C; 2.) 13 h, 21 deg C; | A 80% B 20% C 20% |

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| With C34H43IrN3(1+)*F6P(1-); deuterium In dichloromethane at -78℃; for 16h; Reagent/catalyst; Inert atmosphere; | 96% |
| With Crabtree's catalyst; water-d2; deuterium In dichloromethane at 20℃; | |
| With silver tetrafluoroborate; bis(1,5-cyclooctadiene)diiridium(I) dichloride; deuterium; triphenylphosphine In dichloromethane at 20℃; | 2 mg |
| With water-d2; Ir(cod)(hfac) In N,N-dimethyl acetamide at 160℃; for 0.0333333h; microwave irradiation; | |
| With 2,6-(2,6-iPr2-C6H3-4,5-H2-imidazol-2-ylidene)2C5H3NFe(N2)2; deuterium In tetrahydrofuran at 45℃; under 3040.2 Torr; for 24h; |
-
-
12121-06-1
tris(1,3-diphenyl-1,3-propanedionato)aquoeuropium(III)

-
-
611-74-5
N,N-dimethylbenzamide

| Conditions | Yield |
|---|---|
| In acetone Eu complex in acetone reacted with dimethylbenzamide; solvent evapd., ppt. washed with water, vac. dried; elem. anal.; | 96% |
-
-
288-32-4
1H-imidazole

-
-
611-74-5
N,N-dimethylbenzamide

-
-
1402844-74-9
N-((1H-imidazol-1-yl)methyl)-N-methylbenzamide

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; iron(II) chloride In chlorobenzene at 120℃; for 3h; Inert atmosphere; Schlenk technique; | 96% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; potassium iodide at 80℃; for 6h; Schlenk technique; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: C54H62N6; N,N-dimethylbenzamide With trichlorophosphate In 1,2-dichloro-ethane at 20 - 40℃; for 2h; Vilsmeier-Haack Formylation; Inert atmosphere; Stage #2: With potassium hexafluorophosphate; sodium hydrogencarbonate In water | 96% |
N,N-Dimethylbenzamide Consensus Reports
Reported in EPA TSCA Inventory.
N,N-Dimethylbenzamide Specification
The N,N-Dimethylbenzamide, with the CAS registry number 611-74-5, is also known as Benzamide, N,N-dimethyl-. It belongs to the product categories of Amide; Amides; Carbonyl Compounds; Organic Building Blocks. Its EINECS registry number is 210-279-2. This chemical's molecular formula is C9H11NO and molecular weight is 149.19. What's more, its IUPAC name is the same with its product name.
Physical properties about N,N-Dimethylbenzamide are: (1)ACD/LogP: 0.44; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.43; (4)ACD/LogD (pH 7.4): 0.43; (5)ACD/BCF (pH 5.5): 1.26; (6)ACD/BCF (pH 7.4): 1.26; (7)ACD/KOC (pH 5.5): 41.07; (8)ACD/KOC (pH 7.4): 41.07;(9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 20.31 Å2; (13)Index of Refraction: 1.529; (14)Molar Refractivity: 44.63 cm3; (15)Molar Volume: 144.7 cm3; (16)Surface Tension: 37.4 dyne/cm; (17)Density: 1.03 g/cm3; (18)Flash Point: 111.9 °C; (19)Enthalpy of Vaporization: 49.36 kJ/mol; (20)Boiling Point: 256.1 °C at 760 mmHg; (21)Vapour Pressure: 0.0157 mmHg at 25 °C.
Preparation of N,N-Dimethylbenzamide: this chemical can be prepared by Benzoyl chloride with Dimethylamine. This reaction needs reagent Et3N and solvent diethyl ether. The yield is 90 %.
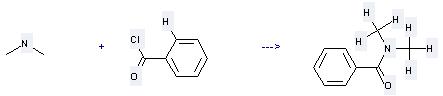
Uses of N,N-Dimethylbenzamide: (1) it is used for deoxidizing reaction of secondary alcohol; (2) it is used to produce other chemicals. For example, it can produce Benzyl-dimethyl-amine. The reaction occurs with reagents HSiEt3, Et2NH and solvent toluene at temperature of 100 °C. The yield is 99.5 %.
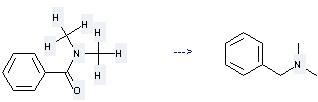
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. If swallowed, it's harmful to health. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(N(C)C)c1ccccc1
(2) InChI: InChI=1S/C9H11NO/c1-10(2)9(11)8-6-4-3-5-7-8/h3-7H,1-2H3
(3) InChIKey: IMNDHOCGZLYMRO-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 960mg/kg (960mg/kg) | Toxicology and Applied Pharmacology. Vol. 19, Pg. 20, 1971. |
Related Products
- N-[(10-Oxido-9,10-dihydro-9-oxa-10-phosphaphenanthrene)methyl]-1,3,5-triazine-2,4,6-triamine
- N10-(Trifluoroacetyl)pteroic acid
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-3-amine
- N-[1,1'-Biphenyl]-4-yl-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide
- N'-[(1,1-Dimethylethoxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N'-methyl-L-lysine
- N1,1-Diphenyl-1,2-ethanediamine
- N-(1,2-Dimethylpropyl)-2-pyridinamine
- N<sup xmlns="">1</sup>-(3,4-DIMETHYL-5-ISOXAZOLYL)SULFANIL-AMIDE LITHIUM SALT
- 611-75-6
- 61175-77-7
- 61177-45-5
- 6117-80-2
- 611-79-0
- 6117-91-5
- 61183-61-7
- 61189-99-9
- 61190-10-1
- 611-91-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View