-
Name
Octanoyl chloride
- EINECS 203-891-6
- CAS No. 111-64-8
- Article Data18
- CAS DataBase
- Density 0.961 g/cm3
- Solubility Soluble in ether
- Melting Point -63 °C
- Formula C8H15ClO
- Boiling Point 195.2 °C at 760 mmHg
- Molecular Weight 162.66
- Flash Point 79.9 °C
- Transport Information UN 3265 8/PG 2
- Appearance Clear liquid
- Safety 26-36/37/39-45-25
- Risk Codes 34-22
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 1-Octanoicacid chloride;Caprylic acid chloride;Capryloyl chloride;Caprylyl chloride;Octanoic acid chloride;n-Octanoyl chloride;BRN 0635917;CCRIS 5990;
- PSA 17.07000
- LogP 3.11230
Synthetic route

| Conditions | Yield |
|---|---|
| With thionyl chloride for 10h; Reflux; | 95.6% |
| With phosgene; propionyl chloride at 50℃; for 12h; Reagent/catalyst; Temperature; | 93.2% |
| With 1,2,3-Benzotriazole; thionyl chloride In dichloromethane at 20℃; Substitution; | 92% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In chloroform Heating; |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide In dichloromethane for 3h; Heating; | |
| In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 2.33333h; Inert atmosphere; | |
| In dichloromethane at 20℃; for 3h; Inert atmosphere; |
-
-
69151-13-9
C3Cl2(C3H7)2

-
-
124-07-2
Octanoic acid

-
A
-
877675-72-4
2,3-diisopropylcyclopropenone

-
B
-
111-64-8
n-octanoic acid chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.0833333h; Inert atmosphere; | A 70.7 mg B n/a |


| Conditions | Yield |
|---|---|
| With thionyl chloride at 50℃; for 1.5h; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In benzene at 0 - 20℃; Acylation; | 100% |
| With tin(IV) chloride; benzene | |
| With carbon disulfide; aluminium trichloride |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; tributylphosphine; copper; zinc In acetonitrile for 1h; Product distribution; Ambient temperature; reactions of other acid chlorides; solvent-effect; effect of var. metals; | 100% |
| With tri-n-butyl-tin hydride In 1-methyl-pyrrolidin-2-one at 20℃; Inert atmosphere; | 96% |
| With pumice stone; platinum at 195℃; under 80 - 90 Torr; Hydrogenation; |
-
-
104089-16-9
2-ethoxycarbonyethylzinc iodide

-
-
111-64-8
n-octanoic acid chloride

-
-
22769-72-8
4-Oxoundecansaeure-ethylester

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl acetamide; benzene at 60℃; for 0.5h; various co-solvents; | 100% |
| tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl acetamide; benzene at 60℃; for 0.5h; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
94594-90-8
(2R)-bornane-10,2-sultam

-
-
141341-55-1
N-octanoyl-(1S)-(-)-10,2-camphorsultam

| Conditions | Yield |
|---|---|
| Stage #1: (2R)-bornane-10,2-sultam With sodium hydride In toluene; mineral oil at 0 - 20℃; for 0.5h; Inert atmosphere; Stage #2: n-octanoic acid chloride In toluene; mineral oil at 0 - 20℃; for 27h; Inert atmosphere; | 100% |
| With dmap; triethylamine In tetrahydrofuran at -5 - 0℃; for 0.5h; Large scale; | 100% |
| With dmap; triethylamine In tetrahydrofuran at 0℃; for 1h; Acylation; | 98.6% |
| With sodium hydride 1) toluene, rt, 2 h, 2) toluene, rt, 2 h; Yield given. Multistep reaction; | |
| With dmap; triethylamine In tert-butyl methyl ether at 20℃; for 1h; | 99.7 %Chromat. |
-
-
2637-34-5
2-Mercaptopyridine

-
-
111-64-8
n-octanoic acid chloride

-
-
89397-99-9
S-(pyridin-2-yl)octanethioate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; Cooling with ice bath; | 100% |
| With TEA In dichloromethane at 0℃; | |
| Stage #1: 2-Sulfanylpyridine With triethylamine In dichloromethane at 0℃; Stage #2: n-octanoic acid chloride at 20℃; for 1.5h; |
-
-
111-64-8
n-octanoic acid chloride


| Conditions | Yield |
|---|---|
| With potassium carbonate In water; ethyl acetate for 2h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium carbonate In acetone at 20℃; for 0.0833333h; | 100% |
| With sodium carbonate; palladium diacetate In acetone at 20℃; for 0.0833333h; Suzuki reaction; | 98% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In benzene at 0 - 20℃; Acylation; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
503856-46-0
(2-amino-4-methyl-benzyl)-phosphonic acid diethyl ester

-
-
303040-42-8
(4-methyl-2-octanoylamino-benzyl)-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2.5h; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
503856-53-9
(6-amino-benzo[1,3]dioxol-5-ylmethyl)-phosphonic acid diethyl ester

-
-
303040-44-0
(6-octanoylamino-benzo[1,3]dioxol-5-ylmethyl)-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 2.5h; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
532987-05-6
N-[(1R)-2-hydroxy-1-(3-methoxyphenyl)ethyl]octanamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane at 0℃; for 2h; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
526217-28-7
N-[(1S,2S)-2-hydroxy-1-(3-methoxyphenyl)propyl]octanamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In tetrahydrofuran; 1,4-dioxane at 0℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 2h; | 99% |
| With pyridine In diethyl ether | 68% |
| With dmap; triethylamine In dichloromethane at 0 - 20℃; | 66% |
| With triethylamine In dichloromethane at 0℃; for 1h; |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 12h; | 100% |
| With pyridine for 12h; | 99% |
-
-
111-64-8
n-octanoic acid chloride

-
-
69884-06-6
4-hydroxy-6-methoxy-(2H)-1,4-benzoxazin-3(4H)-one

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 12h; | 100% |
-
-
94594-90-8
(1S)-(-)-2,10-camphorsultam

-
-
111-64-8
n-octanoic acid chloride

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 0℃; for 1h; | 100% |
-
-
593-51-1
methylamine hydrochloride

-
-
111-64-8
n-octanoic acid chloride

-
-
1119-57-9
N-methyloctanoylamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 5℃; for 2.5h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 3h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
114900-83-3
1-(1H-pyrrol-3-yl)octan-1-one

-
-
1314253-27-4
1-(5-octanoyl-1H-pyrrol-3-yl)octan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: n-octanoic acid chloride With aluminum (III) chloride In 1,2-dichloro-ethane at 25℃; for 0.166667h; Inert atmosphere; Stage #2: 1-(1H-pyrrol-3-yl)octan-1-one In 1,2-dichloro-ethane at 25℃; for 2h; Inert atmosphere; regioselective reaction; | 100% |
-
-
103-67-3
benzyl-methyl-amine

-
-
111-64-8
n-octanoic acid chloride

-
-
1315320-39-8
N-benzyl-N-methyloctanamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20℃; for 20h; | 100% |
-
-
54615-17-7
O-methyl-N-(4-nitrobenzyl)hydroxylamine

-
-
111-64-8
n-octanoic acid chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 100% |
| With pyridine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 100% |

-
-
111-64-8
n-octanoic acid chloride

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20℃; for 20h; | 100% |
-
-
24566-81-2
4-bromo-n-butan-1-amine hydrobromide

-
-
111-64-8
n-octanoic acid chloride

-
-
1619235-29-8
N-(4-bromobutyl)octanamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-n-butan-1-amine hydrobromide With sodium carbonate In dichloromethane; water at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: n-octanoic acid chloride In dichloromethane; water at 0 - 20℃; for 4h; Inert atmosphere; | 100% |
-
-
14502-76-2
6-bromo-1-aminohexane hydrobromide

-
-
111-64-8
n-octanoic acid chloride

-
-
1619235-33-4
N-(6-bromohexyl)octanamide

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1-aminohexane hydrobromide With sodium carbonate In dichloromethane; water at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: n-octanoic acid chloride In dichloromethane; water at 0 - 20℃; for 4h; Inert atmosphere; | 100% |
-
-
5003-71-4
3-bromopropylamine hydrochloride

-
-
111-64-8
n-octanoic acid chloride

-
-
1152510-70-7
N-(3-bromopropyl)octanamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromopropylamine hydrochloride With sodium carbonate In dichloromethane; water at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: n-octanoic acid chloride In dichloromethane; water at 0 - 20℃; for 4h; Inert atmosphere; | 100% |
-
-
543731-34-6
4-((methoxyamino)methyl)benzonitrile

-
-
111-64-8
n-octanoic acid chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 1h; Inert atmosphere; | 100% |

-
-
111-64-8
n-octanoic acid chloride

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20℃; for 7h; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Reflux; | 100% |
| In chloroform at 70℃; for 4h; | 100% |
-
-
111-64-8
n-octanoic acid chloride

-
-
845821-10-5
[3-(3-hydroxypropylamino)propyl]carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 5 - 20℃; for 3.58333h; | 100% |
Octanoyl chloride Specification
The Octanoyl chloride with CAS registry number of 111-64-8 is also known as Caprylic acid chloride. The IUPAC name and product name are the same. It belongs to product categories of Acid Chlorides. Its EINECS registry number is 203-891-6. In addition, the formula is C8H15ClO and the molecular weight is 162.66. This chemical is a clear liquid ang should be sealed in ventilated, cool and dry place away from fire and heat.
Physical properties about Octanoyl chloride are: (1)ACD/LogP: 3.65; (2)ACD/LogD (pH 5.5): 3.65; (3)ACD/LogD (pH 7.4): 3.65; (4)ACD/BCF (pH 5.5): 347.1; (5)ACD/BCF (pH 7.4): 347.1; (6)ACD/KOC (pH 5.5): 2291.2; (7)ACD/KOC (pH 7.4): 2291.2; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 6; (10)Index of Refraction: 1.433; (11)Molar Refractivity: 43.99 cm3; (12)Molar Volume: 169 cm3; (13)Surface Tension: 29.4 dyne/cm; (14)Density: 0.961 g/cm3; (15)Flash Point: 79.9 °C; (16)Enthalpy of Vaporization: 43.14 kJ/mol; (17)Boiling Point: 195.2 °C at 760 mmHg; (18)Vapour Pressure: 0.426 mmHg at 25 °C.
Preparation of Octanoyl chloride: it is prepared by reaction of octanoic acid. The reaction needs reagents SOCl2, benzotriazole and solvent CH2Cl2 at the temperature of 20 °C. The yield is about 92%.

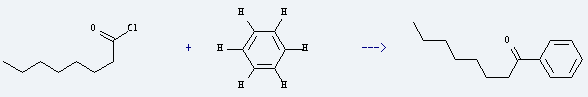
Uses of Octanoyl chloride: it is used to produce 1-phenyl-octan-1-one by reaction with benzene. The reaction occurs with reagent SbF5 intercalated in graphite at 80 °C for 6 hours. The yield is about 40%.
When you are using this chemical, please be cautious about it. As a chemical, it is harmful if swallowed and may cause burns. During using it, wear suitable protective clothing, gloves and eye/face protection. Avoid contact with eyes. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCCCCCCC(=O)Cl
2. InChI: InChI=1S/C8H15ClO/c1-2-3-4-5-6-7-8(9)10/h2-7H2,1H3
3. InChIKey: REEZZSHJLXOIHL-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View