-
Name
Ofloxacin
- EINECS 680-263-1
- CAS No. 82419-36-1
- Article Data15
- CAS DataBase
- Density 1.48 g/cm3
- Solubility Soluble in acetic acid or water. Slightly soluble in methanol
- Melting Point 270-275 °C
- Formula C18H20FN3O4
- Boiling Point 571.5 °C at 760 mmHg
- Molecular Weight 361.373
- Flash Point 299.4 °C
- Transport Information
- Appearance Off-white solid
- Safety 26-36/37/39-24/25-22-37/39
- Risk Codes 22-42/43-68-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Exocin;Floxin (TN);HOE 280;(+/-)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid;Tarivid;DL-8280;Visiren;Prestwick_600;DL 8280;Ocuflox;OFX;Ofloxacin (JP14/USP);ORF 18489;Floxin;OFLX;7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid,9-fluoro-2,3-dihydro-3- methyl-10-(4-methyl-1-piperazinyl)-7-oxo-;Ofloxacin (COS);Levoflxacin;
- PSA 75.01000
- LogP 1.54690
Synthetic route
-
-
109-01-3
1-methyl-piperazine

-
-
82419-35-0
9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 60℃; for 6h; Time; Solvent; | 95.8% |
| With nano iron oxide on ZrO2 coated sulfonic acid In water for 0.416667h; Reflux; | 89% |
| at 150℃; Microwave irradiation; | 83% |
-
-
109-01-3
1-methyl-piperazine

-
-
82419-34-9
ethyl 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylate

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With acetic acid In water; N,N-dimethyl-formamide at 70 - 105℃; for 10h; Temperature; Reagent/catalyst; Solvent; | 87.9% |
-
-
139535-20-9
8-Fluoro-3-methyl-9-(4-methyl-piperazin-1-yl)-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid acetoxymethyl ester

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With water at 37℃; pH 1.8-10; half-life times; |
-
-
139535-21-0
8-Fluoro-3-methyl-9-(4-methyl-piperazin-1-yl)-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid 1-acetoxy-ethyl ester

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With water at 37℃; pH 1.8-10; half-life times; |
-
-
139535-22-1
8-Fluoro-3-methyl-9-(4-methyl-piperazin-1-yl)-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid 1-ethoxycarbonyloxy-ethyl ester

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With water at 37℃; pH 1.8-10; half-life times; |
-
-
115551-33-2
2-hydroxy 3,4-difluoro aniline

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: 80 percent / ethanol / 1 h / Heating 2.1: 95 percent / NaH in oil,; LiClO4 / 18 h / 40 °C 3.1: 80 percent / triphenylphosphine,; DEAD / ethyl acetate / 5 h / 20 °C 4.1: Ac2O,; H2SO4 / 0.75 h / 50 °C 4.2: H2O / 15 h / Heating 5.1: dimethylsulfoxide / 2 h / 100 °C View Scheme |
-
-
85741-74-8
diethyl 2-(((3,4-difluoro-2-hydroxyphenyl)amino)methylene)malonate

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 95 percent / NaH in oil,; LiClO4 / 18 h / 40 °C 2.1: 80 percent / triphenylphosphine,; DEAD / ethyl acetate / 5 h / 20 °C 3.1: Ac2O,; H2SO4 / 0.75 h / 50 °C 3.2: H2O / 15 h / Heating 4.1: dimethylsulfoxide / 2 h / 100 °C View Scheme |
-
-
144298-04-4
(3,4-difluorophenyl)-carbamic acid-tert-butyl ester

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 85 percent 2.1: 80 percent / ethanol / 1 h / Heating 3.1: 95 percent / NaH in oil,; LiClO4 / 18 h / 40 °C 4.1: 80 percent / triphenylphosphine,; DEAD / ethyl acetate / 5 h / 20 °C 5.1: Ac2O,; H2SO4 / 0.75 h / 50 °C 5.2: H2O / 15 h / Heating 6.1: dimethylsulfoxide / 2 h / 100 °C View Scheme |
-
-
86760-99-8
diethyl [(-)-7,8-difluoro-3-methyl-2,3-dihydro-4H-[1,4]benzoxazin-4-yl]methylenemalonate

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: Ac2O,; H2SO4 / 0.75 h / 50 °C 1.2: H2O / 15 h / Heating 2.1: dimethylsulfoxide / 2 h / 100 °C View Scheme | |
| Multi-step reaction with 3 steps 1: PPE / 1 h / 140 - 145 °C 2: 94 percent / conc. HCl / acetic acid / 3 h / Heating 3: 62 percent / dimethylsulfoxide / 12 h / 100 - 140 °C View Scheme |
-
-
124409-86-5
diethyl [3,4-difluoro-2-(2-hydroxypropyloxy)anilinyl]methylenemalonate

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 80 percent / triphenylphosphine,; DEAD / ethyl acetate / 5 h / 20 °C 2.1: Ac2O,; H2SO4 / 0.75 h / 50 °C 2.2: H2O / 15 h / Heating 3.1: dimethylsulfoxide / 2 h / 100 °C View Scheme |
-
-
771-69-7
2,3,4-trifluoronitrobenzene

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 29 percent / 10percent KOH / H2O; dimethylsulfoxide / 2 h / 18 - 20 °C 2: 65 percent / K2CO3, KI / acetone / 4 h / Heating 3: 90 percent / H2, Raney Ni / ethanol 4: 1 h / 140 - 145 °C 5: PPE / 1 h / 140 - 145 °C 6: 94 percent / conc. HCl / acetic acid / 3 h / Heating 7: 62 percent / dimethylsulfoxide / 12 h / 100 - 140 °C View Scheme |
-
-
82419-32-7
3,4-difluoro-2-(2-oxopropyloxy)nitrobenzene

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 90 percent / H2, Raney Ni / ethanol 2: 1 h / 140 - 145 °C 3: PPE / 1 h / 140 - 145 °C 4: 94 percent / conc. HCl / acetic acid / 3 h / Heating 5: 62 percent / dimethylsulfoxide / 12 h / 100 - 140 °C View Scheme |
-
-
82419-26-9
2,3-difluoro-6-nitrophenol

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 65 percent / K2CO3, KI / acetone / 4 h / Heating 2: 90 percent / H2, Raney Ni / ethanol 3: 1 h / 140 - 145 °C 4: PPE / 1 h / 140 - 145 °C 5: 94 percent / conc. HCl / acetic acid / 3 h / Heating 6: 62 percent / dimethylsulfoxide / 12 h / 100 - 140 °C View Scheme |
-
-
82419-34-9
ethyl 9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylate

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 94 percent / conc. HCl / acetic acid / 3 h / Heating 2: 62 percent / dimethylsulfoxide / 12 h / 100 - 140 °C View Scheme | |
| Multi-step reaction with 2 steps 1: acetic acid; sulfuric acid / water / 4 h / Reflux 2: triethylamine / dimethyl sulfoxide / 90 °C View Scheme |
-
-
82419-33-8
(-)-7,8-difluoro-2,3-dihydro-3-methyl-4H-1,4-benzoxazine

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 1 h / 140 - 145 °C 2: PPE / 1 h / 140 - 145 °C 3: 94 percent / conc. HCl / acetic acid / 3 h / Heating 4: 62 percent / dimethylsulfoxide / 12 h / 100 - 140 °C View Scheme |
-
-
109-01-3
1-methyl-piperazine

-
-
82419-35-0
9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide; triethylamine In hydrogenchloride; methanol; diethyl ether; chloroform; dimethyl sulfoxide |

-
-
109-01-3
1-methyl-piperazine

-
-
82419-35-0
9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid


-
B
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| In ethanol; dimethyl sulfoxide |

-
-
109-01-3
1-methyl-piperazine

-
-
119-91-5
2,2'-biquinoline

-
-
6148-64-7
ethyl potassium malonate

-
-
94695-48-4
2,3,4,5-tetrafluorobenzoyl chloride

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; n-butyllithium In tetrahydrofuran; hexane; water; ethyl acetate |


-
-
82419-35-0
9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid

-
-
82419-36-1
ofloxacin

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene / 30 - 60 °C 2: hydrogenchloride / toluene; water / pH 7 3: potassium fluoride / N,N-dimethyl-formamide / Reflux 4: acetic acid; sulfuric acid / water / 4 h / Reflux 5: triethylamine / dimethyl sulfoxide / 90 °C View Scheme |

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: ammonia / toluene / 6 h / 50 °C 2: toluene / 30 - 60 °C 3: hydrogenchloride / toluene; water / pH 7 4: potassium fluoride / N,N-dimethyl-formamide / Reflux 5: acetic acid; sulfuric acid / water / 4 h / Reflux 6: triethylamine / dimethyl sulfoxide / 90 °C View Scheme |

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogenchloride / toluene; water / pH 7 2: potassium fluoride / N,N-dimethyl-formamide / Reflux 3: acetic acid; sulfuric acid / water / 4 h / Reflux 4: triethylamine / dimethyl sulfoxide / 90 °C View Scheme |
-
-
113933-52-1
ethyl 2-(2,3,4,5-tetrafluorobenzoyl)-3-(1-hydroxyprop-2-ylamino)acrylate

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium fluoride / N,N-dimethyl-formamide / Reflux 2: acetic acid; sulfuric acid / water / 4 h / Reflux 3: triethylamine / dimethyl sulfoxide / 90 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-piperazine; C17H14BF2NO8 With triethylamine In dichloromethane at 40℃; for 1.5h; Inert atmosphere; Stage #2: With hydrogenchloride In methanol; water at 0 - 30℃; for 3h; | 7.32 g |
-
-
82419-35-0
9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido[1,2,3-de][1,4]-benzoxazine-6-carboxylic acid

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 1.75 h / 75 - 115 °C / Inert atmosphere 1.2: 3 h / 90 °C 2.1: triethylamine / dichloromethane / 1.5 h / 40 °C / Inert atmosphere 2.2: 3 h / 0 - 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: ofloxacin With sodium hydroxide In ethanol for 0.666667h; Stage #2: cerium(IV) sulphate In ethanol at 20℃; for 24h; Stage #3: water | 98% |


| Conditions | Yield |
|---|---|
| Stage #1: ofloxacin With sodium hydroxide In ethanol for 0.666667h; Stage #2: titanium(IV) sulfate In ethanol at 20℃; for 24h; Stage #3: water | 95% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Reflux; | 92.6% |

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With tetramethylammonium trifluoromethanethiolate In dichloromethane at 20℃; for 1h; | 92% |

| Conditions | Yield |
|---|---|
| In water at 90℃; for 0.5h; pH=7; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: ofloxacin With sodium hydroxide In ethanol for 0.666667h; Stage #2: yttrium(III) chloride In ethanol at 20℃; for 24h; Stage #3: water | 88% |

-
-
66-71-7
1,10-Phenanthroline

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Reflux; | 84.88% |

-
-
82419-36-1
ofloxacin

-
-
530-62-1
1,1'-carbonyldiimidazole

| Conditions | Yield |
|---|---|
| In acetonitrile Reflux; | 82.7% |
| In N,N-dimethyl-formamide at 80 - 90℃; | |
| In N,N-dimethyl-formamide at 80 - 90℃; |
-
-
82419-36-1
ofloxacin

-
-
1211989-50-2
(+/-)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylhydrazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In water for 16h; Reflux; | 82% |
| Multi-step reaction with 2 steps 1: sulfuric acid / Reflux 2: hydrazine hydrate / ethanol / 4 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid / 20 h / Reflux 2: hydrazine hydrate / ethanol / 4 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium hydroxide In methanol pH=7.5; | 82% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Reflux; | 81.22% |


| Conditions | Yield |
|---|---|
| In methanol; ethanol for 24h; Reflux; | 80% |

| Conditions | Yield |
|---|---|
| In methanol for 0.5h; | 79% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium hydroxide at 20℃; for 24h; pH=7.5; | 79% |


| Conditions | Yield |
|---|---|
| In methanol; ethanol for 24h; Reflux; | 78% |
-
-
64-17-5
ethanol

-
-
82419-36-1
ofloxacin

-
-
107884-32-2
ethyl (+/-)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate

| Conditions | Yield |
|---|---|
| With sulfuric acid for 48h; Reflux; | 75% |
| With sulfuric acid Reflux; | |
| With sulfuric acid for 20h; Reflux; |

| Conditions | Yield |
|---|---|
| In methanol; ethanol for 24h; Reflux; | 75% |
-
-
10025-99-7
potassium tetrachloroplatinate(II)

-
-
82419-36-1
ofloxacin

-
-
1161829-26-0, 1162693-99-3
[PtCl2(ofloxacin)]

| Conditions | Yield |
|---|---|
| In water aq. suspn. of ligand added to aq. soln. of Pt compd. (1:1 molar ratio), stirred at room temp. for 48 h; filtered off, washed (water, MeOH), dried, elem. anal.; | 71.9% |
-
-
82419-36-1
ofloxacin

-
-
870-08-6
dioctyltin(IV) oxide

| Conditions | Yield |
|---|---|
| In methanol; benzene Reflux; Inert atmosphere; | 70% |

| Conditions | Yield |
|---|---|
| In methanol; ethanol for 24h; Reflux; | 70% |

-
-
7732-18-5
water

-
-
82419-36-1
ofloxacin

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 2h; pH=7; Reflux; | 70% |


| Conditions | Yield |
|---|---|
| In methanol for 0.5h; | 69% |

-
-
625-56-9
Chloromethyl acetate

-
-
82419-36-1
ofloxacin

-
-
139535-20-9
8-Fluoro-3-methyl-9-(4-methyl-piperazin-1-yl)-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid acetoxymethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 12h; Ambient temperature; | 68.8% |
-
-
82419-36-1
ofloxacin

-
-
818-08-6
di(n-butyl)tin oxide

| Conditions | Yield |
|---|---|
| In methanol; benzene Reflux; Inert atmosphere; | 68% |
-
-
82419-36-1
ofloxacin

-
-
56-35-9
bis(tri-n-butyltin)oxide

| Conditions | Yield |
|---|---|
| In methanol; benzene Reflux; Inert atmosphere; | 68% |

| Conditions | Yield |
|---|---|
| In methanol; ethanol for 24h; Reflux; | 65% |
-
-
82419-36-1
ofloxacin

-
-
108468-00-4
tert-butyl 4-(aminomethyl)benzylcarbamate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 1h; | 65% |
-
-
67-56-1
methanol

-
-
82419-36-1
ofloxacin

-
-
108224-82-4
methyl 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylate

| Conditions | Yield |
|---|---|
| With sulfuric acid Reflux; | 64.5% |
| With sulfuric acid for 20h; Reflux; | 23% |
Ofloxacin History
Ofloxacin Specification
1. Introduction of Ofloxacin
Ofloxacin is one kind of white or almost powder or off-white solid. The Systematic (IUPAC) name of this chemical is (RS)-7-fluoro-2-methyl-6-(4-methylpiperazin-1-yl)-10-oxo-4-oxa-1-azatricyclo[7.3.1.05,13]trideca-5(13),6,8,11-tetraene-11-carboxylic acid. Besides, Ofloxacin belongs to Active Pharmaceutical Ingredients;Ofloxacin;Antibiotic Explorer;Intermediates & Fine Chemicals;Pharmaceuticals;1694 Pharmaceuticals&Personal Care Products;Alphabetic;EPA;NeatsAnalytical Standards;O;Peptide Synthesis/Antibiotics;Pharmaceutical intermediate.
The Classification Code of Ofloxacin is Anti-Bacterial Agents; Anti-Infective Agents; Anti-infective agents, urinary; Antibacterial; Drug / Therapeutic Agent; Enzyme Inhibitors; Human Data; Mutation data; Nucleic Acid Synthesis Inhibitors; Renal Agents; Reproductive Effect. Ofloxacin can slightly soluble in water, alcohol, dichloromethane, and methyl alcohol; sparingly soluble in chloroform. Storage of OFLOXACIN: 1. Store at 2-8 °C; 2. Keep container tightly closed.
2. Properties of Ofloxacin
Physical properties about Ofloxacin are:
(1)XLogP3: -0.4; (2)H-Bond Donor: 1; (3)H-Bond Acceptor: 8; (4)Molecular Formula: C18H20FN3O4; (5)Formula Weight: 361.37; (6)mp: 270-275°C; (7)storage temp.: 2-8°C; (8)Physical State of Ofloxacin: off-white solid.
3. Structure Descriptors of Ofloxacin
(1)InChI: InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)
(2)InChIKey: GSDSWSVVBLHKDQ-UHFFFAOYSA-N
(3)Canonical SMILES: CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O
(4)Smiles: CC1COc2c3n1cc(c(=O)c3cc(c2N4CCN(CC4)C)F)C(=O)O
4. Toxicity of Ofloxacin
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | > 70mg/kg (70mg/kg) | Drugs in Japan Vol. -, Pg. 291, 1995. | |
| dog | LD50 | oral | > 200mg/kg (200mg/kg) | GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Chemotherapy Vol. 32(Suppl, |
| man | TDLo | oral | 17mg/kg/3D-I (17mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" BEHAVIORAL: EXCITEMENT BLOOD: CHANGES IN CELL COUNT (UNSPECIFIED) | Internal Medicine. Vol. 34, Pg. 872, 1995. |
| man | TDLo | oral | 51428ug/kg (51.428mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | American Journal of Medicine. Vol. 87, Pg. 479, 1989. |
| monkey | LD50 | oral | 500mg/kg (500mg/kg) | Chemotherapy Vol. 32(Suppl, | |
| mouse | LD50 | intramuscular | > 600mg/kg (600mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Zhongguo Kangshengsu Zazhi. Chinese Journal of Antibiotics. Vol. 18, Pg. 218, 1993. |
| mouse | LD50 | intraperitoneal | 805mg/kg (805mg/kg) | Ensho. Japanese Journal of Inflammation. Vol. 11, Pg. 343, 1991. | |
| mouse | LD50 | intravenous | 208mg/kg (208mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: COMA | Chemotherapy Vol. 32(Suppl, |
| mouse | LD50 | oral | 3266mg/kg (3266mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Zhongguo Kangshengsu Zazhi. Chinese Journal of Antibiotics. Vol. 18, Pg. 218, 1993. |
| mouse | LDLo | subcutaneous | 7690mg/kg (7690mg/kg) | Chemotherapy Vol. 32(Suppl, | |
| rat | LD50 | intravenous | 273mg/kg (273mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 32(Suppl, |
| rat | LD50 | oral | 3590mg/kg (3590mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 32(Suppl, |
| rat | LD50 | subcutaneous | 7070mg/kg (7070mg/kg) | BEHAVIORAL: ATAXIA BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Chemotherapy Vol. 32(Suppl, |
| women | TDLo | oral | 24mg/kg/3D-I (24mg/kg) | BEHAVIORAL: TOXIC PSYCHOSIS BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: IRRITABILITY | Journal of Clinical Psychiatry. Vol. 53, Pg. 137, 1992 |
Or
| 1. | dnd-hmn:lym 80 mg/L | MUREAV Mutation Research. 211 (1989),171. | ||
| 2. | orl-wmn TDLo:24 mg/kg/3D-I:BAH | JCLPDE Journal of Clinical Psychiatry. 53 (1992),137. | ||
| 3. | orl-man TDLo:51,428 µg/kg:GIT | AJMEAZ American Journal of Medicine. 87 (1989),479. | ||
| 4. | orl-rat LD50:3590 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 5. | scu-rat LD50:7070 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 6. | ivn-rat LD50:273 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 7. | orl-mus LD50:5290 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 8. | scu-mus LDLo:7690 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 9. | ivn-mus LD50:208 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 10. | ivn-dog LDLo:100 mg/kg | NKRZAZ Chemotherapy (Tokyo). 32 (Suppl 1)(1984),1084. | ||
| 11. | orl-mky LD50:500& |
5. Safety information of Ofloxacin
Hazard Codes :
Risk Statements : 22-42/43-68-36/37/38
Safety Statements : 26-36/37/39-24/25-22-37/39
1.Do not breathe dust.
2.Avoid contact with skin. S24/25 Avoid contact with skin and eyes.
3.Avoid contact with eyes.
WGK Germany : 3
RTECS : UU8815550
Poison by intravenous route. Moderately toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Human systemic effects: body temperature increase, diarrhea, hallucinations, hypermotility, irritability, psychosis. Mutation data reported. When heated to decomposition it emits toxic fumes of F− and NOx.
6. Physical Properties of Ofloxacin
| Physical Property | Value | Units | Temp (deg C) | Source |
|---|---|---|---|---|
| Melting Point | 254 dec | deg C | EXP | |
| log P (octanol-water) | -0.39 | (none) | EXP | |
| Water Solubility | 2.83E+04 | mg/L | 25 | EST |
| Vapor Pressure | 1.55E-13 | mm Hg | 25 | EST |
| Henry's Law Constant | 4.98E-20 | atm-m3/mole | 25 | EST |
| Atmospheric OH Rate Constant | 1.97E-10 | cm3/molecule-sec | 25 | EST |
7. Uses of Ofloxacin
OFLOXACIN can be used as fluorinated quinolone antibacterial. A synthetic fluoroquinolone (FLUOROQUINOLONES) antibacterial agent that inhibits the supercoiling activity of bacterial DNA GYRASE, halting DNA REPLICATION.
8. Production of Ofloxacin
(1) 2,3,4-trifluoronitrobenzene as the starting material by selective alkaline hydrolysis, etherification, restore, and C2H5OCH=C(COOEt)2 or (CH3)2NCH=C (COOEt)2 condensation ringaggregate, after hydrolysis with acetic acid boron role, and then the introduction of N-methyl-piperazine-derived products.
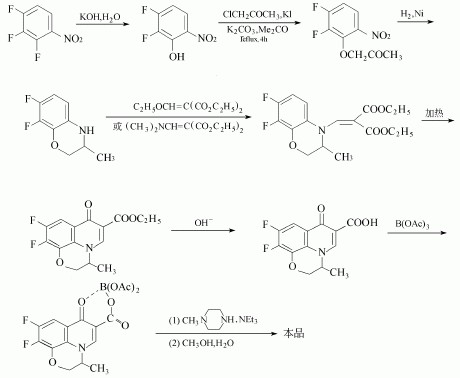
(2) Phthalimide derivative as a raw material generated by fluorination tetrafluorophthalic phthalimide, hydrolysis, decarboxylation of 2,3,4,5-tetrafluoro-benzoic acid, and then chlorinated, acylatingdecarboxylated 2,3,4,5-tetrafluorobenzoyl ethyl acetate, and then the first and of triethyl orthoformate, and after 2-aminopropanol reaction, and then cyclization generated pyridine [1,2,3-de] [1,4] benzo Hey triazine derivatives, and finally reaction of ofloxacin and piperazine.
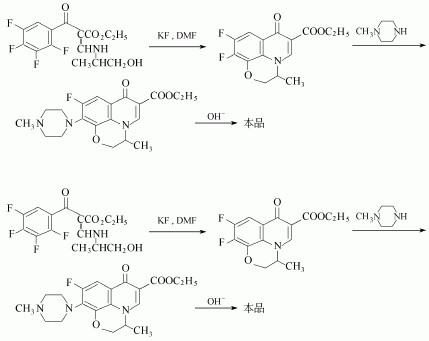
Related Products
- Ofloxacin
- Ofloxacin hydrochloride
- 82420-34-6
- 82424-53-1
- 82424-54-2
- 824-24-8
- 82425-45-4
- 82426-77-5
- 824-27-1
- 82-42-8
- 82428-30-6
- 82436-77-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View