-
Name
Phenacetin
- EINECS 200-533-0
- CAS No. 62-44-2
- Article Data72
- CAS DataBase
- Density 1.099 g/cm3
- Solubility 0.076 g/100 mL in water
- Melting Point 133-136 °C(lit.)
- Formula C10H13NO2
- Boiling Point 355.1 °C at 760 mmHg
- Molecular Weight 179.219
- Flash Point 168.5 °C
- Transport Information
- Appearance white crystalline powder
- Safety 53-45
- Risk Codes 45-22-20/21/22
-
Molecular Structure
-
Hazard Symbols

- Synonyms p-Acetophenetidide(8CI);4-(Acetylamino)phenetole;4-Ethoxy-1-acetylaminobenzene;4-Ethoxyacetanilide;4'-Ethoxyacetanilide;Aceto-4-phenetidine;Acetophenetidin;Acetophenetidine;Acetophenetin;Acetphenetidin;Fenidina;Fenina;Kalmin;N-(4-Ethoxyphenyl)acetamide;N-Acetyl-4-ethoxyaniline;N-Acetyl-p-ethoxyaniline;N-Acetyl-p-phenetidine;NSC 7651;Pertonal;Phenacetin;Phenacetine;Phenazetin;Phenedina;Phenidin;Phenin;p-Ethoxyacetanilide;
- PSA 38.33000
- LogP 2.11670
Synthetic route

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate In water at 100℃; for 0.166667h; Microwave irradiation; Green chemistry; | 94% |

| Conditions | Yield |
|---|---|
| With water for 0.25h; | 88% |
| With sulfuric acid | |
| With (2S)-N-methyl-1-phenylpropan-2-amine hydrate |

| Conditions | Yield |
|---|---|
| With hydrogen; AV-17-8-Pd anion exchanger In ethanol at 45℃; under 760 Torr; | 87.8% |

| Conditions | Yield |
|---|---|
| With pyridine; ammonium hydroxide; hydrogen sulfide In water | 62% |
| Multi-step reaction with 2 steps 2: phosphorus (V)-chloride; diethyl ether View Scheme |

| Conditions | Yield |
|---|---|
| In aq. acetate buffer at 102℃; for 5h; pH=4 - 5; Large scale; | 94.6% |

| Conditions | Yield |
|---|---|
| In aq. acetate buffer at 95 - 100℃; for 6h; pH=4 - 5; Large scale; | 95% |

| Conditions | Yield |
|---|---|
| In aq. acetate buffer at 95 - 100℃; for 6h; pH=4 - 5; Large scale; | 95.5% |

| Conditions | Yield |
|---|---|
| In aq. acetate buffer at 90 - 93℃; for 6.5h; pH=4 - 5; Large scale; | 94.7% |

| Conditions | Yield |
|---|---|
| sodium methylate In ethylene glycol at 120 - 125℃; for 3h; Conversion of starting material; | 79% |

| Conditions | Yield |
|---|---|
| sodium methylate In ethylene glycol at 120 - 125℃; for 3h; Conversion of starting material; | 75% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 3.16667h; Inert atmosphere; | 88% |
| With potassium carbonate In acetone at 0 - 30℃; for 11h; | 77.2% |
| In dichloromethane at 20℃; |

-
-
62-44-2
4-ethoxyacetanilide

| Conditions | Yield |
|---|---|
| With aluminium trichloride; silica gel; zinc(II) chloride In dichloromethane for 0.075h; Beckmann rearrangement; microwave irradiation; | 93% |
| Stage #1: 1-(4-ethoxy-phenyl)-ethanone oxime With triethylamine In dichloromethane at 20℃; for 0.0833333h; Sealed tube; Stage #2: With potassium hydrogen difluoride In water at 20℃; for 2h; Beckmann Rearrangement; Sealed tube; | 87% |

| Conditions | Yield |
|---|---|
| sodium methylate In ethylene glycol at 106 - 130℃; for 10h; Conversion of starting material; | 59% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 25℃; for 8h; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In acetonitrile at 80℃; for 8h; | 65% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; ethanol | |
| With potassium carbonate In acetone for 48h; Heating; | |
| With potassium carbonate In acetone Reflux; | |
| With potassium carbonate In acetone Reflux; | |
| With potassium hydroxide In ethanol for 24h; Reflux; |
-
-
14040-11-0
tungsten hexacarbonyl

-
-
616-38-6
carbonic acid dimethyl ester

-
-
100-29-8
1-ethoxy-4-nitrobenzene

-
-
62-44-2
4-ethoxyacetanilide

| Conditions | Yield |
|---|---|
| With di(rhodium)tetracarbonyl dichloride; 1,3-bis-(diphenylphosphino)propane; sodium phosphate; sodium iodide In water at 120℃; for 24h; Inert atmosphere; Sealed tube; | 99% |


| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride at 80℃; for 2.5h; | 75% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate for 2.5h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; zinc In dichloromethane at 20℃; for 15h; Acetylation; reduction; | A 55% B 15% |


| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; bis-[(trifluoroacetoxy)iodo]benzene at 20℃; for 2h; regioselective reaction; | 57% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 15h; Sealed tube; Inert atmosphere; | 94% |
-
-
15485-31-1, 97221-16-4
(4-nitrobenzylidene)(4-ethoxyphenyl)amine

-
-
108-24-7
acetic anhydride

-
A
-
62-44-2
4-ethoxyacetanilide

-
B
-
555-16-8
4-nitrobenzaldehdye

| Conditions | Yield |
|---|---|
| With water; sodium dodecyl-sulfate at 25 - 30℃; for 0.0833333h; | A 95% B 91% |


| Conditions | Yield |
|---|---|
| With carbon dioxide unter Abdestillieren des gebildeten Wassers bis auf 165grad; | |
| With sodium acetate; acetic acid at 150℃; | |
| Multi-step reaction with 2 steps 1: hydrogen bromide / water / 0.67 h / 30 - 35 °C / Autoclave; Large scale 2: aq. acetate buffer / 6.5 h / 90 - 93 °C / pH 4 - 5 / Large scale View Scheme | |
| Multi-step reaction with 2 steps 1: phosphoric acid / water / 1 h / 15 - 20 °C / Autoclave; Large scale 2: aq. acetate buffer / 6 h / 95 - 100 °C / pH 4 - 5 / Large scale View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid / water / 1 h / 15 - 20 °C / Autoclave; Large scale 2: aq. acetate buffer / 6 h / 95 - 100 °C / pH 4 - 5 / Large scale View Scheme |
-
-
108-24-7
acetic anhydride

-
-
100-29-8
1-ethoxy-4-nitrobenzene

-
A
-
62-44-2
4-ethoxyacetanilide

-
B
-
156-43-4
4-Ethoxyaniline

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol at 45℃; under 760 Torr; Kinetics; Product distribution; Further Variations:; Catalysts; Reaction partners; |

Phenacetin Consensus Reports
NTP 10th Report on Carcinogens. IARC Cancer Review: Group 2A IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 (1987),p. 310.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 13 (1977),p. 141.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 13 (1977),p. 141.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 (1980),p. 135.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 (1980),p. 135.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 (1980),p. 135.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory.
Phenacetin Specification
Phenacetin, its cas register number is 62-44-2. It also can be called Aceto-p-phenetidin. Phenacetin is a white crystalline powder with the chemical composition C10H13NO2. Phenacetin is an analgesic, once widely used; its use has declined because of its adverse effects. In addition to its pain-reducing properties, it also has been used as a fever-reducer, a treatment for rheumatoid arthritis and a treatment for intercostal neuralgia, a rare disorder that causes pain in the nerves around the ribs.
Physical properties about Phenacetin are: (1)ACD/LogP: 1.655; (2)ACD/LogD (pH 5.5): 1.66; (3)ACD/LogD (pH 7.4): 1.66; (4)ACD/BCF (pH 5.5): 10.67; (5)ACD/BCF (pH 7.4): 10.67; (6)ACD/KOC (pH 5.5): 189.43; (7)ACD/KOC (pH 7.4): 189.44; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 3; (11)Index of Refraction: 1.549; (12)Molar Refractivity: 51.836 cm3; (13)Molar Volume: 163.025 cm3; (14)Polarizability: 20.549 10-24cm3; (15)Surface Tension: 39.0260009765625 dyne/cm; (16)Density: 1.099 g/cm3; (17)Flash Point: 168.531 °C; (18)Enthalpy of Vaporization: 60.016 kJ/mol; (19)Boiling Point: 355.054 °C at 760 mmHg
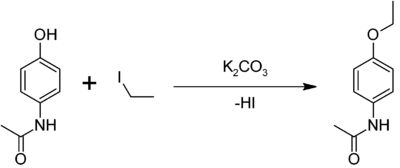
Preparation of Phenacetin: Phenacetin may be synthesized as an example of the Williamson ether synthesis: ethyl iodide, paracetamol, and anhydrous potassium carbonate are refluxed in 2-butanone to give the crude product, which is recrystallized from water.
When you are using this chemical, please be cautious about it as the following:
1. Avoid exposure - obtain special instruction before use;
2. In case of accident or if you feel unwell, seek medical advice immediately (show label where possible);
You can still convert the following datas into molecular structure:
(1)InChI=1S/C10H13NO2/c1-3-13-10-6-4-9(5-7-10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12);
(2)InChIKey=CPJSUEIXXCENMM-UHFFFAOYSA-N;
(3)SmilesCCOc1ccc(cc1)NC(=O)C
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 260mg/kg (260mg/kg) | National Technical Information Service. Vol. PB282-666, | |
| guinea pig | LD50 | oral | 1870mg/kg (1870mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Toxicology and Applied Pharmacology. Vol. 2, Pg. 23, 1960. |
| hamster | LD50 | oral | 1690mg/kg (1690mg/kg) | BEHAVIORAL: COMA BEHAVIORAL: ATAXIA CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP | Pharmazie. Vol. 8, Pg. 572, 1953. |
| man | LDLo | unreported | 74mg/kg (74mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| mouse | LC50 | inhalation | 33900mg/m3 (33900mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 34(10), Pg. 36, 1969. | |
| mouse | LD50 | intraperitoneal | 540mg/kg (540mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 81, Pg. 659, 1961. | |
| mouse | LD50 | oral | 866mg/kg (866mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 28, Pg. 1644, 1978. | |
| mouse | LD50 | subcutaneous | 1625mg/kg (1625mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 8, Pg. 25, 1958. | |
| rabbit | LD50 | oral | 2500mg/kg (2500mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 21(9), Pg. 53, 1977. | |
| rabbit | LD50 | subcutaneous | 1gm/kg (1000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Arzneimittel-Forschung. Drug Research. Vol. 21, Pg. 719, 1971. |
| rat | LD50 | intraperitoneal | 634mg/kg (634mg/kg) | Nippon Yakurigaku Zasshi. Japanese Journal of Pharmacology. Vol. 62, Pg. 11, 1966. | |
| rat | LD50 | oral | 1650mg/kg (1650mg/kg) | Toxicology and Applied Pharmacology. Vol. 1, Pg. 240, 1959. | |
| rat | LD50 | oral | 1650mg/kg (1650mg/kg) | CARDIAC: PULSE RATE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Toxicology and Applied Pharmacology. Vol. 1, Pg. 240, 1959. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View