-
Name
Phenethyl acetate
- EINECS 203-113-5
- CAS No. 103-45-7
- Article Data245
- CAS DataBase
- Density 1.03 g/cm3
- Solubility NEGLIGIBLE
- Melting Point -31 °C
- Formula C10H12O2
- Boiling Point 233.3 °C at 760 mmHg
- Molecular Weight 164.204
- Flash Point 101.7 °C
- Transport Information
- Appearance clear colorless to pale yellow liquid
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols 36/37/38:;
- Synonyms Aceticacid, phenethyl ester (8CI);Phenethyl alcohol, acetate (6CI);2-Phenethylacetate;2-Phenylethyl acetate;Benzylcarbinyl acetate;NSC 71927;Acetic acid,2-phenylethyl ester;Phenylethyl ethanoate;b-Phenethyl acetate;b-Phenylethanol acetate;b-Phenylethyl acetate;
- PSA 26.30000
- LogP 1.79220
Synthetic route

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate at 20℃; for 0.25h; | 100% |
| Stage #1: acetic anhydride With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 0.1h; | 100% |
| With boron trifluoride diethyl etherate In ethyl acetate for 0.00138889h; | 100% |
-
-
506-96-7
Acetyl bromide

-
-
78926-09-7
1-tert-butyldimethylsilyloxy-2-phenylethane

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With tin(II) bromide In dichloromethane for 0.3h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride; TiO(OTf)2 In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 0.3h; Product distribution / selectivity; | 100% |
| Stage #1: acetic anhydride; bis(tetrahydrofurane)oxovanadium(IV) dichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 12h; Product distribution / selectivity; | 99% |
| Stage #1: acetic anhydride; bis(acetylacetonato)dioxidomolybdenum(VI) In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 16h; Product distribution / selectivity; | 98% |

| Conditions | Yield |
|---|---|
| With 1,3-dichlorotetrabutyldistannoxane In toluene for 0.5h; Heating; | 99% |
| With lipase from Candida rugosa at 50℃; for 48h; Enzymatic reaction; | 99.4% |
| With pseudomonas fuorescens lipase immobilized on multiwall carbon nano-tubes at 50℃; for 4h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) for 24h; Ambient temperature; | 99% |
| With Cp2Ti(OSO2C8F17)2 at 80℃; for 2h; Neat (no solvent); | 99% |
| With zirconocene bis(perfluorooctanesulfonate) trihydrate*(tetrahydrofuran) In neat (no solvent) at 80℃; Catalytic behavior; Solvent; Sealed tube; Green chemistry; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With Cp2Ti(OSO2C8F17)2 at 20℃; Neat (no solvent); | 99% |
| zirconium(IV) oxychloride at 20℃; for 0.00833333h; | 98% |
| bismuth(III) oxychloride at 20℃; for 0.00833333h; | 97% |

| Conditions | Yield |
|---|---|
| With 1,3-dichlorotetrabutyldistannoxane for 2h; Heating; | 99% |
| With zirconocene bis(perfluorooctanesulfonate) trihydrate*(tetrahydrofuran) In neat (no solvent) at 65℃; for 5h; Sealed tube; Green chemistry; chemoselective reaction; | 92% |
| With 1-ethyl-3-methylimidazolium acetate at 80℃; for 16h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; | 55.7% |
| With iron(III) trifluoromethanesulfonate at 20℃; for 5h; Schlenk technique; |
-
-
14629-58-4
trimethyl(phenethyloxy)silane

-
-
108-24-7
acetic anhydride

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate In acetonitrile at 20℃; for 0.25h; | 99% |
| With polyvinylpolypyrrolidone-bound boron trifluoride In acetonitrile at 20℃; for 1h; | 97% |
| With alumina supported P2O5 at 20℃; for 0.833333h; neat (no solvent); | 90% |
| Sulfate; titanium(IV) oxide at 20℃; for 0.2h; | 84% |
-
-
60-12-8
2-phenylethanol

-
-
155164-63-9
2-acetyl-4,5-dichloropyridazin-3(2H)-one

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-phenylethanol With aluminum (III) chloride In tetrahydrofuran for 0.5h; Stage #2: 2-acetyl-4,5-dichloropyridazin-3(2H)-one In tetrahydrofuran at 20℃; for 0.166667h; Time; | 99% |
-
-
75-36-5
acetyl chloride

-
-
105966-41-4
1-(tert-butyldiphenylsiloxy)-2-phenylethane

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In acetonitrile for 0.1h; Ambient temperature; | 98% |
| With zinc(II) chloride In acetonitrile for 0.8h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| With 1,3-dichlorotetrabutyldistannoxane for 12h; Heating; | 98% |
| for 6h; Heating; | 97% |
| Stage #1: 2-phenylethanol With potassium tert-butylate In dimethyl sulfoxide at 20℃; for 0.166667h; Inert atmosphere; Stage #2: ethyl acetate In dimethyl sulfoxide at 20℃; for 0.166667h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate for 0.416667h; Heating; | 98% |
-
-
1927-61-3
2-(2-phenylethoxy)tetrahydro-2H-pyran

-
-
64-19-7
acetic acid

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate for 0.5h; Heating; | 98% |
| K5 for 3.5h; Heating; | 95 % Chromat. |

| Conditions | Yield |
|---|---|
| With iron(III) chloride at 80℃; for 16h; Retro-Claisen condensation; Neat (no solvent); | 98% |
| With iron(III) trifluoromethanesulfonate at 80℃; for 10h; Retro-Claisen condensation; Neat (no solvent); | 98% |
| indium(III) triflate at 80℃; for 24h; retro-Claisen condensation; | 95% |
-
-
54673-17-5
[2-(ethoxymethoxy)ethyl]benzene

-
-
108-24-7
acetic anhydride

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With 12-tungstophosphoric acid immobilized on [bmim][FeCl4] at 120 - 130℃; for 0.025h; Microwave irradiation; | 96% |
| With 1-butyl-3-methylimidazolium tetrachloroindate at 145 - 150℃; for 0.0416667h; Microwave irradiation; | 92% |
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.05h; Microwave irradiation; chemoselective reaction; | 90% |
-
-
60-12-8
2-phenylethanol

-
-
108-24-7
acetic anhydride

-
A
-
64-19-7
acetic acid

-
B
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With 3-((3-(trisilyloxy)propyl)propionamide)-1-methylimidazolium chloride ionic liquid supported on magnetic nanoparticle Fe2O3 at 20℃; for 1.33333h; | A n/a B 96% |


| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; bromine; sodium hydrogencarbonate In dichloromethane; water | 95% |
-
-
506-96-7
Acetyl bromide

-
-
7500-77-8
(phenethyloxy)triphenylmethane

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 20℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| indium(III) triflate at 80℃; for 24h; retro-Claisen condensation; | 95% |
| With iron(III) chloride at 80℃; for 16h; Retro-Claisen condensation; Neat (no solvent); | 95% |
-
-
506-96-7
Acetyl bromide

-
-
14629-58-4
trimethyl(phenethyloxy)silane

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With tin(II) bromide In dichloromethane for 0.333333h; Ambient temperature; | 94% |
| With tin(II) bromide In dichloromethane for 0.333333h; Product distribution; Ambient temperature; variation of acetylating agent, Lewis-acid reagent, and time; | 94% |
-
-
60-12-8
2-phenylethanol

-
-
85106-60-1
1-acetyl-3-benzylimidazolium bromide

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| In chloroform for 1h; Ambient temperature; | 94% |
| In chloroform for 1h; Product distribution; Ambient temperature; variation of solvents; | 94% |

| Conditions | Yield |
|---|---|
| zirconium acetylacetonate In acetonitrile for 17h; Ambient temperature; | 94% |
| zirconium acetylacetone In acetonitrile for 17h; Product distribution; Ambient temperature; various catalysts, other alcohols investigated; | 94% |

| Conditions | Yield |
|---|---|
| With acetic anhydride In dichloromethane | 94% |

-
-
1670-46-8
2-acetylcyclopentanaone

-
-
60-12-8
2-phenylethanol

-
A
-
960305-76-4
6-oxo-heptanoic acid phenethyl ester

-
B
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) at 80℃; for 24h; Neat (no solvent); Inert atmosphere; | A 94% B 6% |
| indium(III) triflate at 80℃; for 24h; retro-Claisen condensation; | A 86% B 4% |

-
-
54673-12-0
(2-(methoxymethoxy)ethyl)benzene

-
-
108-24-7
acetic anhydride

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium tetrachloroindate at 145 - 150℃; for 0.0416667h; Microwave irradiation; | 94% |
| With 12-tungstophosphoric acid immobilized on [bmim][FeCl4] at 120 - 130℃; for 0.025h; Microwave irradiation; | 94% |
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.0416667h; Microwave irradiation; chemoselective reaction; | 90% |
-
-
14629-58-4
trimethyl(phenethyloxy)silane

-
-
141-78-6
ethyl acetate

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| With titanium tetrachloride for 1.2h; Heating; | 93% |
-
-
1927-61-3
2-(2-phenylethoxy)tetrahydro-2H-pyran

-
-
108-24-7
acetic anhydride

-
-
103-45-7
acetic acid phenethyl ester

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate In acetonitrile for 0.5h; Heating; | 93% |
| With iron(III) sulfate In 1,2-dichloro-ethane for 3h; Heating; | 82% |
| K5 at 20℃; for 0.25h; | 91 % Chromat. |

| Conditions | Yield |
|---|---|
| With caesium carbonate at 125℃; for 19h; | 93% |

| Conditions | Yield |
|---|---|
| With C. antarctica B immobilized lipase In toluene at 60℃; for 4h; Enzymatic reaction; | 93% |

| Conditions | Yield |
|---|---|
| With acetic anhydride In acetonitrile | 92% |


| Conditions | Yield |
|---|---|
| With phosphate buffer; Phenyl acetate In diethyl ether for 2.75h; Ambient temperature; pig liver acetone powder; | A 18% B 100% |
-
-
103-45-7
acetic acid phenethyl ester

-
-
49681-79-0
O-deuterio-2-phenyl-ethanol

| Conditions | Yield |
|---|---|
| With molybdenum(VI) oxychloride; d(4)-methanol at 20℃; for 27h; | 98% |

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; | 96% |
| With methanol; potassium permanganate at 25℃; chemoselective reaction; | 92% |
| With 2,2-dibutyl-1,3,2-dioxastannane; cesium fluoride In N,N-dimethyl-formamide at 20℃; for 0.5h; | 85% |
Phenethyl acetate Chemical Properties
Molecule structure of Phenethyl acetate (CAS NO.103-45-7):
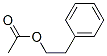
IUPAC: Phenethyl acetate
Molecular Formula: C10H12O2
Molecular Weight: 164.2 g/mol
Density: 1.034 g/cm3
Melting Point: -31 °C
Boiling Point: 233.3 °C at 760 mmHg
Flash Point: 101.7 °C
Water Solubility: negligible
Index of Refraction: 1.501
Molar Refractivity: 46.84 cm3
Molar Volume: 158.7 cm3
Surface Tension: 35.7 dyne/cm
Enthalpy of Vaporization: 47 kJ/mol
Vapour Pressure: 0.0564 mmHg at 25 °C
XLogP3: 2.3
H-Bond Acceptor: 2
Rotatable Bond Count: 4
Exact Mass: 164.08373
MonoIsotopic Mass: 164.08373
Topological Polar Surface Area: 26.3
Heavy Atom Count: 12
Canonical SMILES: CC(=O)OCCC1=CC=CC=C1
InChI: InChI=1S/C10H12O2/c1-9(11)12-8-7-10-5-3-2-4-6-10/h2-6H,7-8H2,1H3
InChIKey: MDHYEMXUFSJLGV-UHFFFAOYSA-N
EINECS: 203-113-5
Product Categories: Miscellaneous
Phenethyl acetate Uses
Phenethyl acetate (CAS NO.103-45-7) is an important intermediate. It is also used in the preparation of rose, jasmine and hyacinth fragrance.
Phenethyl acetate Production
Phenethyl acetate is made by the reaction of benzene ethanol and acetic anhydride in the presence of sodium acetate .
Phenethyl acetate Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | FCTXAV Food and Cosmetics Toxicology. 12 (1974),957. | ||
| 2. | orl-rat LD50:3670 mg/kg | VPITAR Voprosy Pitaniya. Problems of Nutrition. 33 (5)(1974),48. | ||
| 3. | orl-mus LD50:3670 mg/kg | VPITAR Voprosy Pitaniya. Problems of Nutrition. 33 (5)(1974),48. | ||
| 4. | orl-gpg LD50:3670 mg/kg | VPITAR Voprosy Pitaniya. Problems of Nutrition. 33 (5)(1974),48. | ||
| 5. | skn-rbt LD50:6210 mg/kg | FCTXAV Food and Cosmetics Toxicology. 12 (1974),807. |
Phenethyl acetate Consensus Reports
Reported in EPA TSCA Inventory.
Phenethyl acetate Safety Profile
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25
S24/25:Avoid contact with skin and eyes.
WGK Germany: 1
RTECS: AJ2220000
HS Code: 29153990
Moderately toxic by ingestion. Mildly toxic by skin contact. A skin irritant. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, and dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Phenethyl acetate Specification
Phenethyl acetate (CAS NO.103-45-7) is also named as 2-Phenethyl acetate ; 2-Phenylethyl acetate ; 4-06-00-03073 (Beilstein Handbook Reference) ; AI3-03878 ; Acetic acid, 2-phenylethyl ester ; Acetic acid, phenethyl ester ; BRN 0638179 ; Benzylcarbinyl acetate ; Ethanol, 2-phenyl-, acetate ; FEMA No. 2857 ; NSC 71927 ; Phenethyl acetate (natural) ; Phenethyl alcohol, acetate ; UNII-67733846OW ; beta-Phenylethyl acetate . Phenethyl acetate (CAS NO.103-45-7) is clear colorless to pale yellow liquid with sweet smell. It is insoluble in water, soluble in ethanol, ether and other organic solvents.
Related Products
- Phenethyl 2-methylbutyrate
- Phenethyl acetate
- Phenethyl alcohol
- Phenethyl benzoate
- Phenethyl butyrate
- Phenethyl caffeate
- Phenethyl chloracetate
- Phenethyl chloride
- Phenethyl cinnamate
- PHENETHYL ISOBUTYRATE
- 10345-73-0
- 1034574-30-5
- 103457-72-3
- 103458-58-8
- 1034614-73-7
- 103466-73-5
- 103467-50-1
- 103-46-8
- 103468-74-2
- 1034706-81-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View