-
Name
Phenethyl alcohol
- EINECS 200-456-2
- CAS No. 60-12-8
- Article Data1023
- CAS DataBase
- Density 1.02 g/cm3
- Solubility 20 g/L (20 °C) in water
- Melting Point -27 °C(lit.)
- Formula C8H10O
- Boiling Point 218.199 °C at 760 mmHg
- Molecular Weight 122.167
- Flash Point 98.4 °C
- Transport Information UN 2810 6.1/PG 3
- Appearance colorless liquid
- Safety 26-28-36/37-36/37/39
- Risk Codes 21/22-36/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Phenethylalcohol (8CI);(2-Hydroxyethyl)benzene;2-Phenethanol;2-Phenethyl alcohol;2-Phenyl-1-ethanol;Benzyl carbinol;Ethanol, 2-phenyl-;NSC 406252;PEA;Phenethanol;b-(Hydroxyethyl)benzene;b-PEA;b-Phenethanol;b-Phenethyl alcohol;b-Phenylethanol;b-Phenylethyl alcohol;
- PSA 20.23000
- LogP 1.22140
Synthetic route

| Conditions | Yield |
|---|---|
| With morpholine-borane; boron trifluoride diethyl etherate In diethyl ether for 2h; Product distribution; Ambient temperature; | 100% |
| With ammonium formate; palladium on activated charcoal In methanol for 2h; Heating; | 100% |
| With hydrogen In methanol at 25℃; under 750.075 Torr; Reagent/catalyst; Flow reactor; regioselective reaction; | 100% |
-
-
14629-58-4
trimethyl(phenethyloxy)silane

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With Dowex 1-X8 In ethanol for 8h; Ambient temperature; | 100% |
| With bismuth(lll) trifluoromethanesulfonate In methanol at 20℃; for 0.0166667h; | 98% |
| With methanol; 1,3-disulfonic acid imidazolium hydrogen sulfate at 20℃; for 0.0833333h; Green chemistry; | 98% |
-
-
78926-09-7
1-tert-butyldimethylsilyloxy-2-phenylethane

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In methanol at 23℃; for 3.5h; | 100% |
| With water; scandium tris(trifluoromethanesulfonate) In acetonitrile for 1h; Ambient temperature; | 98% |
| sulfonic acid functionalized nanoporous silica In methanol at 35℃; for 1.5h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; hydrogen In ethanol at 40℃; under 7600.51 Torr; for 19h; Solvent; Autoclave; Inert atmosphere; | 100% |
| With magnesium sulfate In tetrahydrofuran; dichloromethane | 92% |
| With magnesium sulfate In tetrahydrofuran; dichloromethane | 92% |

| Conditions | Yield |
|---|---|
| With phosphate buffer; Phenyl acetate In diethyl ether for 2.75h; Ambient temperature; pig liver acetone powder; | A 18% B 100% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; silica gel In hexane for 3h; Heating; | 100% |
| With methanol; sodium tetrahydroborate In diethyl ether at 20℃; for 38h; Reduction; | 96% |
| With sodium tetrahydroborate In diethylene glycol dimethyl ether at 104℃; | 95% |
-
-
14629-62-0
1-(triethylsiloxy)-2-phenylethane

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With iron(III) chloride In methanol at 23℃; for 0.0833333h; | 100% |
| With methanol; trimethylsilyl bromide at 20℃; for 0.166667h; chemoselective reaction; | 98% |
| With Selectfluor In acetonitrile at 150℃; for 0.05h; Microwave irradiation; | 82% |
| With iron(III) p-toluenesulfonate hexahydrate In methanol at 20℃; for 0.333333h; | 80% |
| With hydrogenchloride In methanol at 20℃; for 16h; |
-
-
1093198-50-5
C24H20O3

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With (triphenylphosphine)gold(I) chloride; silver trifluoromethanesulfonate In ethanol; benzene at 20℃; for 0.3h; | 100% |
-
-
501014-38-6
allyl 2-phenylethyl carbonate

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| [RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid In methanol at 30℃; for 0.5h; | 99% |
| With Fe3O4@SiO2-[(4-(5-O3Si-pentylcarbamoyl)-2-pyridinecarboxylato)CpRu(η3-C3H5)]PF6 In methanol at 30℃; for 2h; Inert atmosphere; chemoselective reaction; | 99.9% |
| [RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid In methanol at 30℃; for 0.5h; Product distribution; Further Variations:; Solvents; | 99 % Spectr. |
-
-
100-41-4
ethylbenzene

-
A
-
123-07-9
4-Ethylphenol

-
B
-
98-85-1, 13323-81-4
1-Phenylethanol

-
C
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With rabbit liver microsomal cytochrome P-450 In water at 25℃; for 12h; | A 0.13% B 99.8% C 0.08% |


| Conditions | Yield |
|---|---|
| With [carbonylchlorohydrido{bis[2-(diphenylphosphinomethyl)ethyl]amino}ethylamino] ruthenium(II); potassium tert-butylate; hydrogen In toluene at 75℃; under 37503.8 Torr; for 24h; Catalytic behavior; Pressure; Temperature; Reagent/catalyst; regioselective reaction; | A 99% B n/a |
| With lithium triethylborohydride In tetrahydrofuran at 0℃; for 0.0833333h; Product distribution; | A 97% B 3% |
| With Li(1+)*C12H28AlO3(1-) In tetrahydrofuran; hexane at 0℃; for 0.17h; Yields of byproduct given; | A 95% B n/a |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; tri-n-butyl-tin hydride for 2h; Product distribution; Ambient temperature; different aldehydes, reagents, reaction temperature and time; | 99% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide; tri-n-butyl-tin hydride for 2h; Ambient temperature; | 99% |
| With hydrogen In water at 60℃; under 22502.3 Torr; for 0.00611111h; Flow reactor; Green chemistry; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 5h; Glovebox; Autoclave; | 99% |
| 95% | |
| With lithium borohydride In diethyl ether; toluene at 100℃; for 1h; | 92% |
-
-
14289-65-7
2-(2-propenyloxy)ethylbenzene

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With quinoline-2-carboxylic acid; cyclopentadienylruthenium(II) trisacetonitrile hexafluorophosphate In methanol at 30℃; for 3h; | 99% |
| [RuCp(η3-C3H5)(QA)]PF6, QA=quinaldic acid In methanol at 30℃; for 3h; | 99% |
| quinoline-2-carboxylic acid; cyclopentadienylruthenium(II) trisacetonitrile hexafluorophosphate In methanol; dichloromethane at 30℃; for 0.5h; Conversion of starting material; | 99% |

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride; hafnium tetrachloride In tetrahydrofuran at -20℃; for 3h; Inert atmosphere; | 99% |
| With (Ppyz)Zr(BH4)2Cl2 In diethyl ether for 8h; Heating; | 92% |
| With phenylsilane; potassium tert-butylate; water; sodium triethylborohydride; cobalt(II) chloride In 1,4-dioxane; toluene at 60℃; for 15h; Inert atmosphere; Glovebox; Schlenk technique; | 84% |

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With Oxone; water In acetone at 20℃; for 0.0333333h; | 99% |

| Conditions | Yield |
|---|---|
| With zinc(II) tetrahydroborate; N,N,N,N,-tetramethylethylenediamine In diethyl ether at 0℃; for 0.5h; | 98% |
| With methyltriphenylphosphonium tetrahydroborate In dichloromethane Reduction; | 98% |
| With Zr(BH4)2Cl2(dabco)2 In tetrahydrofuran for 1.2h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In methanol for 0.05h; Heating; | 98% |
| With dimethylbromosulphonium bromide In methanol; dichloromethane at 20℃; for 0.416667h; | 97% |
| With trichloroisocyanuric acid In methanol at 20℃; for 5h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: isopropyl phenylacetate With diethylzinc; lithium chloride In tetrahydrofuran; hexane at 20℃; for 6h; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; hexane; water at 20℃; for 8h; Catalytic behavior; Concentration; Time; Inert atmosphere; chemoselective reaction; | 98% |
| With phenylsilane; potassium tert-butylate; water; sodium triethylborohydride; cobalt(II) chloride In 1,4-dioxane; toluene at 60℃; for 15h; Inert atmosphere; Glovebox; Schlenk technique; | 93% |
| Stage #1: isopropyl phenylacetate With diethoxymethylane; zinc diacetate In tetrahydrofuran at 65℃; for 24h; Inert atmosphere; Stage #2: With methanol; potassium hydroxide chemoselective reaction; | 98 %Chromat. |
| Stage #1: isopropyl phenylacetate With iron (II) stearate; ethylenediamine In toluene at 20℃; for 0.0833333h; Inert atmosphere; Schlenk technique; Stage #2: In toluene at 100℃; for 20h; Inert atmosphere; Schlenk technique; | 71 %Chromat. |
| With 1,1'-methylene-bis(3-benzyl-1H-imidazol-3-ium) diiodide; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; potassium tert-butylate; hydrogen In 1,4-dioxane at 100℃; under 37503.8 Torr; for 6h; | 50 %Chromat. |
-
-
501014-38-6
allyl 2-phenylethyl carbonate

-
-
75-33-2
2-propanethiol

-
A
-
50996-72-0
allylisopropyl sulfide

-
B
-
124-38-9
carbon dioxide

-
C
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With [Bu4N][Fe(CO)3(NO)]; tris(2,4,6-trimethylphenyl)phosphine In ethanol at 40℃; Inert atmosphere; | A n/a B n/a C 98% |

-
-
34420-17-2
(2-phenylethyl)boronic acid

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With pyrene-1,6-dione; oxygen; isopropyl alcohol at 20℃; under 760.051 Torr; for 40h; Irradiation; Green chemistry; | 98% |
| With rose bengal; triethylamine In ethanol at 25℃; for 12h; Schlenk technique; Irradiation; | 97% |
| With 2,5-dimethylfuran; zinc(II) phthalocyanine; oxygen In tetrahydrofuran at 25℃; under 760.051 Torr; for 2h; Time; Irradiation; Sealed tube; Schlenk technique; | 95% |
-
-
18240-10-3
(+/-)-2-(3-cyclohexenyl)ethanol

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With hydrogen at 250℃; under 750.075 Torr; Reagent/catalyst; Green chemistry; | 97.92% |

| Conditions | Yield |
|---|---|
| With [Zn(BH4)2(py)] In tetrahydrofuran for 1.5h; Heating; | 97% |
| With zinc(II) tetrahydroborate In tetrahydrofuran for 3h; Heating; | 95% |
| With borane-THF In tetrahydrofuran for 3.25h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With iron(III) sulfate; water In toluene at 110℃; for 1h; Ionic liquid; | 97% |
| With sodium carbonate at 160 - 165℃; | |
| With sodium carbonate at 175℃; |

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate In tetrahydrofuran for 1h; Ambient temperature; | A 94% B 97% |
-
-
128702-35-2
N-(4-Phenethyloxymethyl-phenyl)-acetamide

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone | 97% |
-
-
54673-12-0
(2-(methoxymethoxy)ethyl)benzene

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| phosphotungstic acid In ethanol for 3h; Heating; | 97% |
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.025h; Microwave irradiation; chemoselective reaction; | 95% |
| With 1-thiopropane; zinc dibromide In dichloromethane at 20℃; for 0.1h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; formic acid; (η5-cyclopentadienyl) (η6-naphthalene)ruthenium hexafluorophosphate; water In tetrahydrofuran at 55℃; for 48h; | 97% |
| With 1-hydroxytetraphenylcyclopentadienyl(tetraphenyl-2,4-cyclopentadien-1-one)-μ-hydrotetracarbonyldiruthenium(II); Ru(Cp)(PPh2PytBu)2(MeCN)PF6; water In isopropyl alcohol at 70℃; for 48h; Concentration; Reagent/catalyst; Inert atmosphere; regioselective reaction; | 90% |
| With formic acid; F6P(1-)*C16H22N3Ru(1+); water In 1-methyl-pyrrolidin-2-one at 25℃; for 48h; Inert atmosphere; Sealed tube; | 83% |
| Multi-step reaction with 2 steps 1: cyclopentadienylruthenium(II) trisacetonitrile hexafluorophosphate; [2,2]bipyridinyl; formic acid / water; 1-methyl-pyrrolidin-2-one / 24 h / 25 °C / Inert atmosphere; Sealed tube 2: N1,N1-dimethyl-N2-(pyridin-2-ylmethylene)ethane-1,2-diamine; cyclopentadienylruthenium(II) trisacetonitrile hexafluorophosphate; formic acid; water / 1-methyl-pyrrolidin-2-one / 24 h / 25 °C / Inert atmosphere; Sealed tube View Scheme |

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; | 96% |
| With methanol; potassium permanganate at 25℃; chemoselective reaction; | 92% |
| With 2,2-dibutyl-1,3,2-dioxastannane; cesium fluoride In N,N-dimethyl-formamide at 20℃; for 0.5h; | 85% |
-
-
60-12-8
2-phenylethanol

-
-
17376-04-4
2-phenethyl iodide

| Conditions | Yield |
|---|---|
| With 1H-imidazole; iodine; triphenylphosphine In diethyl ether at 0℃; for 1h; | 100% |
| With trimethylsilylphosphate; sodium iodide for 10h; Ambient temperature; | 98% |
| With N-iodosaccharine; triphenylphosphine In dichloromethane at 20℃; for 0.5h; | 95% |

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate at 20℃; for 0.25h; | 100% |
| Stage #1: acetic anhydride With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 0.1h; | 100% |
| With boron trifluoride diethyl etherate In ethyl acetate for 0.00138889h; | 100% |
-
-
60-12-8
2-phenylethanol

-
-
545-06-2
trichloroacetonitrile

-
-
99421-73-5
β-phenylethyl trichloroacetimidate

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane | 100% |
| With sodium | |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; |

| Conditions | Yield |
|---|---|
| With TiO(acac)2 In xylene for 15h; Heating; | 100% |
| With iron(III)-acetylacetonate In 5,5-dimethyl-1,3-cyclohexadiene for 15h; Inert atmosphere; Reflux; | 97% |
| With 4-nitro-diphenylammonium triflate In toluene at 80℃; for 30h; | 95% |
-
-
60-12-8
2-phenylethanol

-
-
145387-82-2
N-(tert-butyloxycarbonyl)(2-trimethylsilylethyl)sulfonamide

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 0℃; for 0.0833333h; | 100% |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl; sodium hypochlorite; sodium chlorite In acetonitrile at 35℃; pH 6.7; | 100% |
| With sodium hypochlorite; sodium chlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In aq. phosphate buffer; water; acetonitrile at 35℃; pH=6.7; Green chemistry; | 100% |
| With oxygen; sodium hydroxide In water at 90℃; for 18h; Catalytic behavior; | 100% |
-
-
75-15-0
carbon disulfide

-
-
60-12-8
2-phenylethanol

-
-
74-88-4
methyl iodide

-
-
70061-62-0
S-methyl O-phenylethyl carbonodithioate

| Conditions | Yield |
|---|---|
| Stage #1: 2-phenylethanol With sodium hydride In tetrahydrofuran; mineral oil for 0.333333h; Stage #2: carbon disulfide In tetrahydrofuran; mineral oil at 20℃; for 0.333333h; Stage #3: methyl iodide In tetrahydrofuran; mineral oil for 0.333333h; | 100% |
| Stage #1: carbon disulfide; 2-phenylethanol With 1H-imidazole; sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 0.5h; | 95% |
| With sodium hydroxide; tetrabutylammomium bromide In water | 75% |
| Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| at 20℃; for 0.0833333h; Neat (no solvent); | 100% |
| With lanthanum(III) nitrate at 20℃; for 0.166667h; | 96% |
| With pyridine In dichloromethane at 25℃; for 5h; Acylation; | |
| With picoline In dichloromethane for 1h; Reflux; | |
| Stage #1: 2-phenylethanol With bis(cyclopentadienyl)titanium dichloride; manganese; diiodomethane In tetrahydrofuran at 20℃; for 2.5h; Inert atmosphere; Stage #2: pivaloyl chloride In tetrahydrofuran at 20℃; for 1.5h; Solvent; Inert atmosphere; |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
60-12-8
2-phenylethanol

-
-
1927-61-3
2-(2-phenylethoxy)tetrahydro-2H-pyran

| Conditions | Yield |
|---|---|
| With H6P2W18O62 In toluene at 20℃; for 2h; | 100% |
| With phosphotungstic acid In toluene at 20℃; for 1h; | 100% |
| With iron(III) sulfate at 20℃; for 1h; | 98% |
-
-
60-12-8
2-phenylethanol

-
-
19360-67-9
4-carboxymethoxy-benzoic acid

| Conditions | Yield |
|---|---|
| With [Cl(C6F13C2H4)2SnOSn(C2H4C6F13)2Cl]2 In various solvent(s) at 150℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| With [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold (I) at 150℃; for 0.5h; Kinetics; Inert atmosphere; Microwave irradiation; | 100% |
| With (triphenylphosphine)gold(I) chloride at 150℃; for 1.5h; Microwave irradiation; Green chemistry; | 96% |
| With n-butyllithium; 2,3,5,6-tetrafluoro-1,4-benzoquinone; chloro-diphenylphosphine In dichloromethane at 20℃; for 3h; Product distribution; Further Variations:; Reagents; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: butanoic acid anhydride With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 0.15h; | 100% |
| With iron(III) p-toluenesulfonate hexahydrate In neat (no solvent) at 20℃; for 1h; | 85% |
-
-
2568-90-3
di-n-butyloxymethane

-
-
60-12-8
2-phenylethanol

-
-
92101-62-7
formaldehyde-(butyl-phenethyl-acetal)

| Conditions | Yield |
|---|---|
| With Nafion-H SAC-13 silica nanocomposite at 100℃; | 100% |
-
-
60-12-8
2-phenylethanol

-
-
1079-66-9
chloro-diphenylphosphine

-
-
849604-79-1
2-phenylethyl diphenylphosphinite

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 1h; | 100% |
| With n-butyllithium In tetrahydrofuran at 0℃; for 1h; | |
| With triethylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | |
| Stage #1: 2-phenylethanol With n-butyllithium In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: chloro-diphenylphosphine In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Schlenk technique; |

| Conditions | Yield |
|---|---|
| at 50℃; for 120h; | 100% |
| Stage #1: 2-phenylethanol; caffeic acid at 20℃; for 0.25h; Molecular sieve; Ionic liquid; Inert atmosphere; Stage #2: With Tocopherol at 130℃; under 760.051 Torr; for 0.15h; Solvent; Reagent/catalyst; Temperature; Molecular sieve; Ionic liquid; Inert atmosphere; Microwave irradiation; | 95% |
| In benzene for 84h; Heating; Dean-Stark trap; | 40% |
-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; diazaphospholidinium-based reagent In acetonitrile at 20℃; for 0.166667h; | 100% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; 3 A molecular sieve; potassium carbonate; [Ru(p-cumene)Cl2]2 In toluene at 110℃; for 24h; Product distribution; Further Variations:; Catalysts; | 100% |
| With 1,1'-bis(diphenylphosphino)ferrocene; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; potassium carbonate In toluene at 20℃; for 24.1667h; Inert atmosphere; Molecular sieve; Reflux; | 88% |
| With C61H45N3OP2RuS; potassium hydroxide In toluene at 110℃; for 12h; Schlenk technique; | 79% |
| Stage #1: 2-phenylethanol With 1,1'-bis-(diphenylphosphino)ferrocene; [RuCl2(p-cymene)(3-INC5H4)] In toluene for 0.166667h; Reflux; Stage #2: tert-butylamine In toluene; acetonitrile for 24h; Reagent/catalyst; Reflux; | |
| Stage #1: 2-phenylethanol With [bis((μ-chloro)chloro(η6-phenylacetic acid)ruthenium(II))] In toluene at 110℃; for 0.166667h; Reflux; Stage #2: tert-butylamine In toluene; acetonitrile at 110℃; for 24h; Catalytic behavior; Reagent/catalyst; Time; Reflux; |
-
-
60-12-8
2-phenylethanol

-
-
754-05-2
ethenyltrimethylsilane

-
A
-
74-85-1
ethene

-
B
-
14629-58-4
trimethyl(phenethyloxy)silane

| Conditions | Yield |
|---|---|
| hydrogenchloride; chlorobis(ethylene)rhodium(I) dimer In 1,4-dioxane; chloroform at 20℃; for 2h; Product distribution / selectivity; | A n/a B 100% |
| chlorobis(cyclooctene)rhodium(I) dimer In toluene at 70℃; for 3h; Product distribution / selectivity; | A n/a B 100% |
| hydrogenchloride; chlorobis(cyclooctene)rhodium(I) dimer In 1,4-dioxane; chloroform at 20℃; for 2h; Product distribution / selectivity; | A n/a B 96% |


| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride; TiO(OTf)2 In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 0.3h; Product distribution / selectivity; | 100% |
| Stage #1: acetic anhydride; bis(tetrahydrofurane)oxovanadium(IV) dichloride In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 12h; Product distribution / selectivity; | 99% |
| Stage #1: acetic anhydride; bis(acetylacetonato)dioxidomolybdenum(VI) In dichloromethane at 20℃; for 0.5h; Stage #2: 2-phenylethanol In dichloromethane at 20℃; for 16h; Product distribution / selectivity; | 98% |
-
-
286371-46-8
4-[(6,7-dimethoxy-4-quinolyl)oxy]-2,5-dimethylaniline

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
60-12-8
2-phenylethanol

-
-
144-55-8
sodium hydrogencarbonate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; dichloromethane; chloroform; toluene | 100% |

-
-
60-12-8
2-phenylethanol

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| With cyclooctadiene ruthenium(II) dichloride; potassium tert-butylate; 1,3-diisopropyl-1H-imidazol-3-ium chloride; tricyclopentylphosphonium tetrafluoroborate In toluene for 24h; Inert atmosphere; Reflux; | 100% |
| With dichloro(1,5-cyclooctadiene)ruthenium(II); potassium tert-butylate; 1,3-diisopropyl-1H-imidazol-3-ium chloride; tricyclopentylphosphonium tetrafluoroborate In toluene at 110℃; for 24h; Inert atmosphere; | 100% |
| With pyridine; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; sodium hydride; 1,3-di(propan-2-yl)-1H-imidazol-3-ium bromide In toluene for 36h; Inert atmosphere; Reflux; | 98% |
Phenethyl alcohol Consensus Reports
Reported in EPA TSCA Inventory.
Phenethyl alcohol Specification
1. Introduction of Phenethyl alcohol
Phenethyl alcohol is colourless liqui, it is an alcohol with a pleasant floral odor. The IUPAC Name of it is 2-phenylethanol.
2. Properties of Phenethyl alcohol
Index of Refraction: 1.535
Molar Refractivity: 37.33 cm3
Molar Volume: 119.7 cm3
Polarizability: 14.8×10-24cm3
Surface Tension: 39.6 dyne/cm
Density: 1.02 g/cm3
Flash Point: 98.4 °C
Enthalpy of Vaporization: 48.05 kJ/mol
Boiling Point: 218.2 °C at 760 mmHg
Vapour Pressure: 0.0741 mmHg at 25°C
Melting Point: −27 °C(lit.)
Water Solubility: 20 g/L (20 ºC)
Stability: Stable. Substances to be avoided include strong acids and strong oxidizing agents. Combustible.
Physical Appearance: colourless liquid
Product Categories: Miscellaneous
Synonyms of Benzeneethanol (CAS NO.60-12-8): 1-Phenyl-2-ethanol ; 2-Hydroxyethylbenzene ; 2-Phenethyl alcohol ; 2-Phenylethanol ; Ethanol, 2-phenyl- ; Methanol, benzyl- ; beta-Fenethylalkohol ; beta-Hydroxyethylbenzene ; beta-Phenylethanol
3. Structure Descriptors of Phenethyl alcohol
InChI=1S/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2
4. Toxicity of Phenethyl alcohol
| 1. | eye-rbt 12 g/10M MLD | ARZNAD Arzneimittel-Forschung. Drug Research. 9 (1959),349. | ||
| 2. | skn-gpg 100 mg MLD | FCTXAV Food and Cosmetics Toxicology. 13 (1975),903. | ||
| 3. | mmo-smc 1000 ppm | GENRA8 Genetical Research. 13 (1969),107. | ||
| 4. | uns-mus:ast 8360 µmol/L | BCPCA6 Biochemical Pharmacology. 22 (1973),2511. | ||
| 5. | orl-rat LD50:1790 mg/kg | FCTXAV Food and Cosmetics Toxicology. 2 (1964),327. | ||
| 6. | scu-mus LDLo:1640 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 14 (1920),211. | ||
| 7. | skn-rbt LD50:790 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 28 (1974),313. | ||
| 8. | orl-rbt LDLo:2 g/kg | JEENAI Journal of Economic Entomology. 48 (1955),139. |
5. Safety Information of Phenethyl alcohol
Moderately toxic by ingestion and skin contact. A skin and eye irritant. Experimental teratogenic effects. Other experimental reproductive effects. Causes severe central nervous system injury to experimental animals. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:
 Xn
Xn Risk Statements: 21/22-36/38
21/22: Harmful in contact with skin and if swallowed
36/38: Irritating to eyes and skin
Safety Statements: 26-28-36/37-36/37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37: Wear suitable protective clothing and gloves
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
RIDADR: UN 2810 6.1/PG 3
WGK Germany: 1
HazardClass: 6.1
PackingGroup: III
6. Preparation of Phenethyl alcohol
Phenethyl alcohol can be made by a number of procedures; the Grignard reaction is used generally:
C6H5Br + Mg → C6H5MgBr
C6H5MgBr + CH2CH2O → C6H5CH2CH2OMgBr
C6H5CH2CH2OMgBr + H+ → C6H5CH2CH2OH
However, the Friedel-Crafts reaction is also employed to manufacture this particular chemical.
C6H6 + CH2CH2O (+ AlCl3) → C6H5CH2CH2OH (+AlCl3)
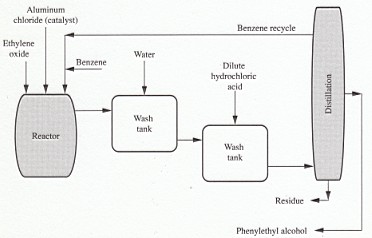
FIGURE 1 Manufacture of phenylethyl alcohol.
7. Use of Phenethyl alcohol
Phenethyl alcohol is much used in perfume formulation. It is used as an additive in cigarettes. It is also used as a preservative in soaps due to its stability in basic conditions. In biology it is of interest due to its antimicrobial properties.
Related Products
- Phenethyl 2-methylbutyrate
- Phenethyl acetate
- Phenethyl alcohol
- Phenethyl benzoate
- Phenethyl butyrate
- Phenethyl caffeate
- Phenethyl chloracetate
- Phenethyl chloride
- Phenethyl cinnamate
- PHENETHYL ISOBUTYRATE
- 60129-59-1
- 60129-60-4
- 60129-64-8
- 6012-97-1
- 60132-35-6
- 60133-17-7
- 60133-18-8
- 60134-26-1
- 601-34-3
- 60137-06-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View