-
Name
Phenyl acetate
- EINECS 204-575-0
- CAS No. 122-79-2
- Article Data474
- CAS DataBase
- Density 1.072 g/cm3
- Solubility miscible with ethanol, ethyl ether, chloroform and acetic acid, hardly soluble in water
- Melting Point 195-196℃
- Formula C8H8O2
- Boiling Point 195.499 °C at 760 mmHg
- Molecular Weight 136.15
- Flash Point 79.73 °C
- Transport Information
- Appearance colourless liquid
- Safety 36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms (Acetyloxy)benzene;Acetoxybenzene;NSC 27795;Phenol acetate;Acetic acid, phenyl ester;o-Acetylphenol;
- PSA 26.30000
- LogP 1.61190
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine; aluminum oxide at 103 - 105℃; for 2h; microwave irradiation; | 100% |
| With 2,6-di-tert-butyl-pyridine; sodium tetracarbonyl cobaltate In acetonitrile for 12h; | 100% |
| With SBA-15-Ph-Pr-SO3H at 20℃; for 0.25h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; Aliquat 336 In neat (no solvent) at 70℃; for 0.5h; | A n/a B 100% |
| With potassium carbonate; Aliquat 336 In neat (no solvent) at 70℃; for 0.5h; Product distribution; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| With carbon monoxide In tetrahydrofuran (N2 or Ar), complex dissolved in THF, evacuated, excess CO introduced at room temp.C for 0.3 h; IR; | A n/a B 100% |
| With carbon monoxide In tetrahydrofuran (N2 or Ar), complex dissolved in THF, evacuated, equimolar amount CO introduced at -78°C for 10 h; IR; | A n/a B 31% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In acetonitrile at 20℃; for 1h; | 99% |
| In cyclohexane at 20℃; Solvent; Temperature; | 99% |
| montmorillonite K-10 In dichloromethane for 2h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With oxone; silica gel In dichloromethane at 20℃; for 16h; Baeyer-Villiger oxidation; | 99% |
| With 3-chloro-benzenecarboperoxoic acid In water at 80℃; for 2h; Oxidation; | 89% |
| With 3-chloro-benzenecarboperoxoic acid In water at 80℃; for 1.5h; | 85% |

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In neat (no solvent) at 70 - 140℃; for 0.183333h; Catalytic behavior; Temperature; Solvent; Reagent/catalyst; Time; Microwave irradiation; | 99% |
| With Rasta resin-(1,5,7-triazabicyclo[4.4.0]dec-5-ene)[RR-TBD] In tetrahydrofuran at 20 - 60℃; | 97% |
| With 4 A molecular sieve; tetrabutylammonium tricarbonylnitrosylferrate In hexane at 80℃; for 24h; | 80% |

| Conditions | Yield |
|---|---|
| With iridium(III) chloride; triphenylphosphine In acetonitrile at 180℃; under 3750.38 Torr; for 20h; Autoclave; | 95% |


| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In neat (no solvent) at 60 - 140℃; for 0.183333h; Microwave irradiation; | 93% |
-
-
82969-01-5
4-acetoxyphenyl tosylate

-
-
122-79-2
Phenyl acetate

| Conditions | Yield |
|---|---|
| With dimethylamine borane; potassium carbonate; tricyclohexylphosphine; bis(triphenylphosphine)nickel(II) chloride In N,N-dimethyl-formamide at 20℃; for 14h; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 12h; Inert atmosphere; Reflux; | 92% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In chloroform at 20℃; for 11h; Baeyer-Villiger oxidation; | A 7% B 91% |
| With methyltrifluoromethyldioxirane; (η5-C5H4SiMe3)2NbH(O)C=CPh2 In tetrahydrofuran at 25℃; | |
| With (η5-C5H4SiMe3)2NbH(O)C=CPh2 at 25℃; | |
| With α,α,α-trifluorotoluene; 3-chloro-benzenecarboperoxoic acid In chloroform for 3.5h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| In acetone | 90% |


| Conditions | Yield |
|---|---|
| With carbon monoxide In diethyl ether (N2 or Ar), complex dissolved in Et2O, evacuated, excess CO introduced at room temp. for 24 h; IR; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| With air; zirconium tetraacetate; cobalt(II) acetate; palladium dichloride In acetylacetone at 105℃; under 7500.6 Torr; for 6h; Product distribution; Activation energy; Further Variations:; Catalysts; Reagents; Pressures; Temperatures; solvents, ratio; | A 89% B n/a |
| With air; zirconium tetraacetate; cobalt(II) acetate; palladium dichloride In acetylacetone at 105℃; under 7500.6 Torr; for 6h; Product distribution; Activation energy; Kinetics; Further Variations:; Catalysts; Reagents; Pressures; Temperatures; solvents, ratio; | A 89% B n/a |


| Conditions | Yield |
|---|---|
| copper(II) sulfate In dichloromethane for 2.5h; Heating; | 89% |
| With 1,4-diaza-bicyclo[2.2.2]octane; bismuth (III) nitrate pentahydrate for 0.05h; Microwave irradiation; | 85% |
| With iron(III) sulfate In 1,2-dichloro-ethane for 1.5h; Heating; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: methoxybenzene With boron tribromide In dichloromethane at 0 - 20℃; Inert atmosphere; Stage #2: acetic acid In dichloromethane at 0 - 20℃; Inert atmosphere; | 88% |
-
-
64-19-7
acetic acid

-
-
100-51-6
benzyl alcohol

-
-
108-95-2
phenol

-
A
-
140-11-4
Benzyl acetate

-
B
-
122-79-2
Phenyl acetate

| Conditions | Yield |
|---|---|
| With poly(4-vinylpyridine) perchlorate In neat (no solvent) at 20℃; for 0.5h; | A 88% B 12% |


| Conditions | Yield |
|---|---|
| With palladium diacetate; bis-[(trifluoroacetoxy)iodo]benzene at 80℃; for 17h; | 88% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 150℃; for 1h; | 87% |
| In neat (no solvent) at 150℃; Microwave irradiation; | 82% |
| Stage #1: phenol With aluminum (III) chloride In tetrahydrofuran at 20℃; for 1h; Stage #2: N-acetyl saccharin In tetrahydrofuran for 1h; Reflux; | 11% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 12h; | 86% |

| Conditions | Yield |
|---|---|
| Sulfate; titanium(IV) oxide at 20℃; for 0.5h; | 85% |
| With polyvinylpolypyrrolidone-bound boron trifluoride In acetonitrile at 20℃; for 3h; | 85% |
| With silver perchlorate; titanium tetrachloride In dichloromethane Ambient temperature; | 62% |

| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.0833333h; | 100% |
| With C17H16BrMnNO3P; potassium tert-butylate; hydrogen In 1,4-dioxane at 100℃; under 37503.8 Torr; for 16h; Autoclave; | 89% |

| Conditions | Yield |
|---|---|
| With HZSM-5(30) In water for 7h; Product distribution; Heating; var. catalysts; other acetylated alcohols; | 100% |
| silica gel; toluene-4-sulfonic acid In water; toluene at 80℃; for 6h; | 100% |
| With sodium hydrogen telluride; acetic acid In ethanol for 0.5h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With dilithium tetra(tert-butyl)zincate at 0℃; for 1h; Inert atmosphere; | 100% |
| With 2Zn(2+)*C20H14N4*4C2H3O2(1-)*1.5CH4O In neat (no solvent) at 50℃; for 18h; | 99% |
| With [Zn(bis(2-pyridylmethyl)amine)2]I2 at 50℃; for 48h; | 100 %Chromat. |

| Conditions | Yield |
|---|---|
| With C12F18O13Zn4 at 90℃; for 18h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With cell-free extract containing recombinant PpATaseCH In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 24h; pH=7.5; Time; | 99% |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; triethylamine In dichloromethane at -20℃; for 1h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| In toluene for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With cell-free extract containing recombinant PpATaseCH In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 24h; pH=7.5; Time; | 98% |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; tris(triphenylphosphine)ruthenium(II) chloride; di-n-butyliodotin hydride; 3-ethyl-1-methyl-1H-imidazol-3-ium 2,2,2-trifluoroacetate In 1,4-dioxane; dimethyl sulfoxide at 20 - 75℃; for 6h; Reagent/catalyst; Inert atmosphere; | 97.4% |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; tris(triphenylphosphine)ruthenium(II) chloride; di-n-butyliodotin hydride; 3-ethyl-1-methyl-1H-imidazol-3-ium 2,2,2-trifluoroacetate In 1,4-dioxane; dimethyl sulfoxide at 20 - 80℃; for 6h; Reagent/catalyst; Inert atmosphere; | 97.3% |

| Conditions | Yield |
|---|---|
| With copper acetylacetonate; tris(triphenylphosphine)ruthenium(II) chloride; di-n-butyliodotin hydride; 3-ethyl-1-methyl-1H-imidazol-3-ium 2,2,2-trifluoroacetate In 1,4-dioxane; dimethyl sulfoxide at 20 - 90℃; for 5h; Reagent/catalyst; Inert atmosphere; | 97.2% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran-d8 at 66℃; for 48h; Catalytic behavior; Temperature; Concentration; | 96% |
| With allylchloro-[1,3-bis(diisopropylphenyl)-imidazole-2-ylidene]palladium(II); water; potassium carbonate In toluene at 110℃; for 16h; Inert atmosphere; | 92% |
| With cell-free extract containing recombinant PpATaseCH In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 24h; pH=7.5; Time; | 70% |

| Conditions | Yield |
|---|---|
| With Lawessons reagent In xylene for 10h; Heating; | A n/a B 96% |

| Conditions | Yield |
|---|---|
| With cell-free extract containing recombinant PpATaseCH In aq. phosphate buffer; dimethyl sulfoxide at 35℃; for 24h; pH=7.5; Concentration; | 96% |

| Conditions | Yield |
|---|---|
| With bromine fluoride In ethanol; chloroform at -78℃; for 0.25h; | 95% |
| With tetrachloromethane; N-Bromosuccinimide | |
| With phosphorus pentabromide | |
| With bromine fluoride; ethanol 1.) CFCl3, -75 deg C; 2.) CHCl3, -75 deg C, 5-15 min; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| for 0.116667h; Rearrangement; microwave irradiation; | 95% |
| With aluminum (III) chloride In neat (no solvent) at 140 - 150℃; Fries Phenol Ester Rearrangement; | 90% |
| With hydrogen fluoride supported on silica gel In neat (no solvent) at 55℃; for 4h; Temperature; Green chemistry; | 77% |
Phenyl acetate Consensus Reports
Phenyl acetate Specification
Phenyl acetate is an organic compound with the formula C8H8O2, and its systematic name is the same with the product name. With the CAS registry number 122-79-2, it is also named as Acetic acid, phenyl ester. It belongs to the product category of Organics. Its EINECS number is 204-575-0. In addition, the molecular weight is 136.15. Its classification code is Skin / Eye Irritant. This substance is used as a solvent and organic synthesis intermediates. This chemical is flammable. When meet high flame, it is easy to burn. It is harmful if swallowed. When using it, you need wear suitable protective clothing.
Physical properties of Phenyl acetate are: (1)ACD/LogP: 1.653; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.65; (4)ACD/LogD (pH 7.4): 1.65; (5)ACD/BCF (pH 5.5): 10.62; (6)ACD/BCF (pH 7.4): 10.62; (7)ACD/KOC (pH 5.5): 188.83; (8)ACD/KOC (pH 7.4): 188.83; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.504; (14)Molar Refractivity: 37.594 cm3; (15)Molar Volume: 127.062 cm3; (16)Polarizability: 14.903×10-24cm3; (17)Surface Tension: 34.88 dyne/cm; (18)Density: 1.072 g/cm3; (19)Flash Point: 79.73 °C; (20)Enthalpy of Vaporization: 43.17 kJ/mol; (21)Boiling Point: 195.499 °C at 760 mmHg; (22)Vapour Pressure: 0.4 mmHg at 25°C.
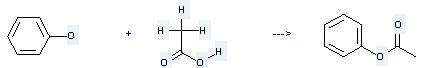
Preparation: this chemical can be prepared by acetic acid and phenol at the ambient temperature. This reaction will need reagents Ph3P, CCl4, Et3N and solvent acetonitrile with the reaction time of 4 hours. The yield is about 97%.
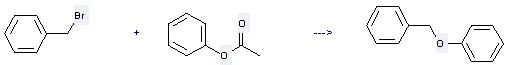
Uses of Phenyl acetate: it can be used to produce benzyloxy-benzene at the ambient temperature. It will need reagent NaOMe and solvent dimethylformamide with the reaction time of 0.5 hour. The yield is about 93%.
You can still convert the following datas into molecular structure:
(1)SMILES: CC(=O)Oc1ccccc1
(2)Std. InChI: InChI=1S/C8H8O2/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
(3)Std. InChIKey: IPBVNPXQWQGGJP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 8mL/kg (8mL/kg) | Union Carbide Data Sheet. Vol. 11/4/1964, | |
| rat | LD50 | oral | 1630uL/kg (1.63mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. |
Related Products
- Phenyl [5-(trifluoromethyl)pyridin-2-yl]carbamate
- Phenyl 2-pyridyl ketoxime
- phenyl 4,5-dimethoxy-2-nitrobenzoate
- Phenyl 4,6-o-benzylidene-1-thio-beta-d-glucopyranoside
- Phenyl 4,6-O-benzylidene-beta-D-glucopyranoside
- Phenyl 4-chloro-1-hydroxy-2-naphthoate
- phenyl acetaldehyde diisobutyl acetal
- Phenyl acetate
- Phenyl acrylate
- Phenyl arsine oxide
- 12279-41-3
- 122795-41-9
- 122795-43-1
- 122799-38-6
- 122799-95-5
- 122799-98-8
- 122800-01-5
- 122-80-5
- 122813-72-3
- 1228175-65-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View