-
Name
Phenyl chloroformate
- EINECS 217-547-8
- CAS No. 1885-14-9
- Article Data79
- CAS DataBase
- Density 1.283 g/cm3
- Solubility hydrolysis in water
- Melting Point -28 °C
- Formula C7H5ClO2
- Boiling Point 186.5 °C at 760 mmHg
- Molecular Weight 156.569
- Flash Point 79 °C
- Transport Information UN 2746 6.1/PG 2
- Appearance colorless liquid with a strong odor
- Safety 26-28-36/37/39-45-28A
- Risk Codes 22-26-34-41-38-29
-
Molecular Structure
-
Hazard Symbols
 T+
T+
- Synonyms Phenoxycarbonyl chloride;Formic acid, chloro-, phenyl ester;4-06-00-00629 (Beilstein Handbook Reference);Fenylester kyseliny chlormravenci [Czech];Fenylester kyseliny chlormravenci;Formic acid, chloro-, phenyl ester (8CI);carbonochloridic acid, phenyl ester;
- PSA 26.30000
- LogP 2.42420
Synthetic route

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0℃; for 2h; | 99% |
| With sodium hydroxide In dichloromethane at 20℃; for 2h; | 88.4% |
| Stage #1: bis(trichloromethyl) carbonate With sodium carbonate; N,N-dimethyl-formamide In toluene at 0℃; for 0.5h; Stage #2: phenol In toluene at 0℃; for 8.5h; | 66% |

| Conditions | Yield |
|---|---|
| With CoCl2 In triphenylphosphine | 91.5% |
| With water Behandeln mit Phosgen und Trichloraethylen; |

| Conditions | Yield |
|---|---|
| 91% |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 100℃; Temperature; | 89.4% |
| With sodium hydroxide; water; toluene at 30℃; bis:>40 degreeC.; | |
| With N,N-dimethyl-aniline; benzene |
-
-
851443-09-9
5-tert-Butoxycarbonylamino-3-methyl-thiophene-2-carboxylic acid ethyl ester

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| Stage #1: 5-tert-Butoxycarbonylamino-3-methyl-thiophene-2-carboxylic acid ethyl ester With trifluoroacetic acid In dichloromethane at 0℃; for 2h; Stage #2: With sodium hydroxide; water In ethyl acetate Product distribution / selectivity; | 89% |
-
-
13509-28-9
S-methyl O-phenyl carbonothioate

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride at 20℃; for 1h; | 78% |

| Conditions | Yield |
|---|---|
| at 30℃; | |
| With toluene |
-
-
861340-55-8
carbonic acid phenyl ester-trichloromethyl ester

-
A
-
75-44-5
phosgene

-
B
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| at 180 - 210℃; |

| Conditions | Yield |
|---|---|
| at 140 - 150℃; im geschlossenen Rohr; | |
| at 140 - 150℃; |


| Conditions | Yield |
|---|---|
| With water |
-
-
456-55-3
1-trifluoromethoxybenzene

-
A
-
102-09-0
bis(phenyl) carbonate

-
B
-
351-80-4
phenyl fluoroformate

-
C
-
1885-14-9
phenyl chloroformate

-
D
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With aluminium trichloride In fluorobenzene at 90℃; for 0.0833333h; |

-
-
4460-46-2
3-chloro-3-phenyl-3H-diazirine

-
A
-
98-88-4
benzoyl chloride

-
B
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With oxygen In solid matrix at -263.2℃; Irradiation; |
-
-
4460-46-2
3-chloro-3-phenyl-3H-diazirine

-
A
-
124-38-9
carbon dioxide

-
B
-
201230-82-2
carbon monoxide

-
C
-
19016-92-3
chlorophenyldiazomethane

-
D
-
98-88-4
benzoyl chloride

-
E
-
1885-14-9
phenyl chloroformate

-
F
-
107135-21-7
1-chlorocyclohepta-1,2,4,6-tetraene

| Conditions | Yield |
|---|---|
| With oxygen In solid matrix at -263.2℃; Product distribution; Mechanism; Irradiation; different O2 concentrations and wavelengths of irradiation; |

-
-
7732-18-5
water

-
-
108-95-2
phenol

-
-
503-38-8
trichloromethyl chloroformate

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| Reaktion von Natriumphenolat (2 Mol); |

-
-
503-38-8
trichloromethyl chloroformate

-
A
-
102-09-0
bis(phenyl) carbonate

-
B
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| je nach den angewandten Mengenverhaeltnissen; |

-
-
4460-46-2
3-chloro-3-phenyl-3H-diazirine

-
A
-
98-88-4
benzoyl chloride

-
B
-
108-90-7
chlorobenzene

-
C
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With oxygen at -247.2℃; under 150 - 200 Torr; Product distribution; Mechanism; Irradiation; study of the oxygen trapping of phenylchlorocarbene intermediate formed from phenylchlorodiazirine in oxigen-doped matrix by irradiation, mechanism is discussed; |


| Conditions | Yield |
|---|---|
| With pyrographite; triethylamine In tetrahydrofuran at 120℃; for 3h; Condensation; | |
| With dmap In toluene at 20℃; for 24h; | |
| With dmap In toluene at 20℃; for 24h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: DBU / tetrahydrofuran / 1 h / 20 °C / 750.06 Torr 1.2: 25 percent / tetrahydrofuran / 2 h / 20 °C / 750.06 Torr 2.1: 78 percent / SO2Cl2 / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: S; DBU / tetrahydrofuran / 6 h / 80 °C / 7500.6 Torr 1.2: 49 percent / tetrahydrofuran / 16 h / 20 °C / 750.06 Torr 2.1: 78 percent / SO2Cl2 / 1 h / 20 °C View Scheme |
-
-
75-44-5
phosgene

-
-
6303-21-5
hypophosphorous acid

-
-
108-95-2
phenol

-
A
-
102-09-0
bis(phenyl) carbonate

-
B
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| In Diphenylmethane |

-
-
51719-70-1
phenyl oxalyl chloride

-
-
10444-89-0
2-amino-5-trifluoromethyl-1,3,4-thiadiazole

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With triethylamine | |
| With triethylamine |
-
-
13932-55-3
phenyl carbonic acid

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With thionyl chloride for 2h; Reflux; Cooling with ice; | |
| With thionyl chloride In toluene for 1h; Reflux; |
-
-
1449331-31-0
2-oxo-2-(pyren-1-yl)ethyl phenyl carbonate

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water / acetonitrile / pH 7.5 / Photolysis; Inert atmosphere 2: sodium carbonate / toluene / 6 h / 0 °C View Scheme |
-
-
104-94-9
4-methoxy-aniline

-
-
1885-14-9
phenyl chloroformate

-
-
20950-96-3
(4-methoxyphenyl)carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With pyridine In ethyl acetate at 20℃; for 1h; | 100% |
| With sodium hydrogencarbonate In tetrahydrofuran; water at 0 - 20℃; for 0.0833333h; | 97% |
| With sodium carbonate In tetrahydrofuran; water; ethyl acetate at 0 - 20℃; | 92% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 23℃; for 4h; Inert atmosphere; | 100% |
| With pyridine In dichloromethane for 3h; | 90% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 3h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2.5h; | 100% |
| With triethylamine In dichloromethane at 0℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 16h; Inert atmosphere; | 100% |
| With pyridine In dichloromethane at 23℃; Cooling with ice; Inert atmosphere; | 99% |
| With pyridine; dmap In dichloromethane at 0 - 20℃; for 13.5h; | 95% |
-
-
613-94-5
benzoic acid hydrazide

-
-
1885-14-9
phenyl chloroformate

-
-
15081-44-4
1-benzoyl-2-phenoxycarbonylhydrazine

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran 1) -10 deg C, 30 min, 2) 1 h, room temperature; | 100% |
| In benzene |
-
-
617-94-7
1-methyl-1-phenylethyl alcohol

-
-
1885-14-9
phenyl chloroformate

-
-
53933-09-8
2-phenylisopropyloxycarbonyl phenyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at -5 - 0℃; | 100% |
| With pyridine In dichloromethane |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane at 20℃; for 1h; | 100% |
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane at 0℃; for 2h; | 75% |
| With sodium hydride In N,N-dimethyl-formamide at 70℃; for 20h; | 52% |
-
-
128425-87-6
6,7-Dimethoxy-1-(3-pentyl)-3,4-dihydroisoquinoline

-
-
1885-14-9
phenyl chloroformate

-
-
128425-88-7
Phenyl 6,7-Dimethoxy-1-(3-pentylidene)-1,2,3,4-tetrahydroisoquinoline-2-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; benzene Heating; | 100% |
-
-
75-86-5
2-hydroxy-2-methylpropanenitrile

-
-
1885-14-9
phenyl chloroformate

-
-
130312-20-8
1-cyano-1-methyl-ethylphenylcarbonate

| Conditions | Yield |
|---|---|
| With pyridine for 0.5h; | 100% |
-
-
72-18-4
L-valine

-
-
1885-14-9
phenyl chloroformate

-
-
126147-70-4
(2S)-3-Methyl-2-(phenoxycarbonyl)aminobutyric acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium hydrogencarbonate for 1.5h; Ambient temperature; | 100% |
| With aluminum oxide; lithium chloride; lithium hydroxide In water at -20 - -10℃; for 5h; pH=9.5 - 10.2; Schotten-Baumann Reaction; Inert atmosphere; | 92% |
| Stage #1: L-valine; phenyl chloroformate With sodium hydroxide In water at 0℃; for 5h; Stage #2: With hydrogenchloride In water pH=2; | 75.9% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
1885-14-9
phenyl chloroformate

-
-
152899-11-1
2,2,2-trifluoroethyl phenyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine | 100% |
| With pyridine In dichloromethane at 20℃; for 1h; |
-
-
62443-00-9
4-(Tetradecyloxy)-benzyl alcohol

-
-
1885-14-9
phenyl chloroformate

-
-
147219-70-3
phenyl <4-(tetradecyloxy)phenyl>methyl carbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane 1.) 0 deg C, 15 min, 2.) RT, 30 min; | 100% |
| With pyridine In dichloromethane |
-
-
78469-77-9, 81076-11-1, 128820-40-6, 78513-03-8
(4S,5S)-4-benzyloxymethyl-5-hydroxymethyl-2,2-dimethyl-1,3-dioxolane

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 100% |
-
-
86485-18-9
2'-methyl-2',3'-dihydrospiro

-
-
1885-14-9
phenyl chloroformate

-
-
86485-19-0
N-phenoxycarbonyl-2',3'-dihydrospiro

| Conditions | Yield |
|---|---|
| In dichloromethane Ambient temperature; overnight; | 100% |
| In dichloromethane; ethyl acetate | 2.6 g (69.7%) |
-
-
74852-62-3
4-[4-(4-methoxy-phenyl)-piperazin-1-yl]-phenylamine

-
-
1885-14-9
phenyl chloroformate

-
-
74853-06-8
phenyl (4-(4-(4-methoxyphenyl)piperazin-1-yl)phenyl)carbamate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 100% |
| With pyridine In dichloromethane at 0 - 20℃; for 2h; | 92.1% |
| With pyridine for 3h; | 90% |
-
-
1885-14-9
phenyl chloroformate

-
-
116970-37-7
3-O-<<2-(dimethylamino)ethyl>carbamoyl>-2-O-methyl-1-O-(octadecylcarbamoyl)glycerol

-
-
116953-42-5
3-O--N-(phenoxycarbonyl)carbamoyl>-2-O-methyl-1-O-(octadecylcarbamoyl)glycerol

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 4h; Ambient temperature; | 100% |
-
-
61210-52-4
(trimethylsilyl)ethynylmagnesium bromide

-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| In dichloromethane at -20℃; | 100% |


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 22℃; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
223545-26-4
(R)-2-((R)-2-Methyl-pent-4-enyl)-2,3-dihydro-1H-pyridin-4-one

-
-
223545-28-6
(R)-2-((R)-2-Methyl-pent-4-enyl)-4-oxo-3,4-dihydro-2H-pyridine-1-carboxylic acid phenyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium | 100% |
| Stage #1: (R)-2-((R)-2-Methyl-pent-4-enyl)-2,3-dihydro-1H-pyridin-4-one With n-butyllithium Inert atmosphere; Stage #2: phenyl chloroformate Inert atmosphere; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
35989-06-1
3-amino-6-(3-pyridyl)pyridine

-
-
264617-08-5
[2,3']bipyridinyl-5-yl-carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 18h; Condensation; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
264617-06-3
2-chloro-3-(3-pyridyl)aniline

-
-
264617-22-3
(2-chloro-3-pyridin-3-yl-phenyl)-carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 18h; Condensation; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
264616-98-0
3-(4-methyl-3-pyridyl)aniline

-
-
264617-09-6
[3-(4-methyl-pyridin-3-yl)-phenyl]-carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 18h; Condensation; | 100% |
-
-
1885-14-9
phenyl chloroformate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 18h; Condensation; | 100% |
-
-
762-04-9
phosphonic acid diethyl ester

-
-
1885-14-9
phenyl chloroformate

-
-
132916-41-7
phenyl diethoxyphosphorylformate

| Conditions | Yield |
|---|---|
| at 120℃; for 1.5h; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
436155-90-7
1-amino-6,8-dimethoxy-3,4-dihydroquinolin-2(1H)-one

-
-
156141-49-0
phenyl (6,8-dimethoxy-2-oxo-1,2,3,4-tetrahydroquinolin-1-yl)carbamate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane at 20℃; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
174004-33-2
trans-2-(2-Vinylstyryl)pyrrole

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutyl-ammonium chloride In dichloromethane for 0.25h; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
285984-25-0
3-tert-butyl-1-p-tolyl-1H-pyrazol-5-amine

-
-
285984-47-6
[5-tert-butyl-2-(4-methylphenyl)-2H-pyrazol-3-yl]carbamic acid phenyl ester

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 100% |
| With sodium hydroxide In water; ethyl acetate at 10.9 - 25.5℃; for 1h; | 95% |
| With sodium carbonate In Isopropyl acetate; water at 20℃; | 88% |
-
-
1885-14-9
phenyl chloroformate

-
-
220496-61-7
(3R,4S,5S,6R)-4-((E)-1,5-Dimethyl-hexa-1,4-dienyl)-5-methoxy-1-oxa-spiro[2.5]octan-6-ol

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0℃; | 100% |
-
-
1885-14-9
phenyl chloroformate

-
-
654055-80-8
1-((3R,4S,5S,6R)-6-Hydroxy-5-methoxy-1-oxa-spiro[2.5]oct-4-yl)-ethanone O-benzyl-oxime

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0℃; | 100% |
-
-
149-30-4
2-thioxo-3H-1,3-benzothiazole

-
-
1885-14-9
phenyl chloroformate

-
-
61588-20-3
S-(1,3-benzothiazol-2-yl) O-phenyl thiocarbonate

| Conditions | Yield |
|---|---|
| With triethylamine In benzene at 20℃; for 1h; | 100% |
Phenyl chloroformate Consensus Reports
Phenyl chloroformate Standards and Recommendations
Phenyl chloroformate Specification
The Phenyl chlorocarbonate, with the CAS registry number 1885-14-9 and EINECS registry number 217-547-8, has the systematic name of phenyl carbonochloridate. And the molecular formula of this chemical is C16H36ClN. It is a kind of clear liquid with a strong odor, and belongs to the following product categories: Miscellaneous; Organics; Chloroformates. In addition, it is sensitive to moisture, and should be stored in the refrigerator. Besides, it is used as reagents for organic synthesis.
The physical properties of Phenyl chlorocarbonate are as following: (1)ACD/LogP: 2.66; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.66; (4)ACD/LogD (pH 7.4): 2.66; (5)ACD/BCF (pH 5.5): 62.02; (6)ACD/BCF (pH 7.4): 62.02; (7)ACD/KOC (pH 5.5): 667.87; (8)ACD/KOC (pH 7.4): 667.87; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.532; (14)Molar Refractivity: 37.81 cm3; (15)Molar Volume: 121.9 cm3; (16)Polarizability: 14.98×10-24cm3; (17)Surface Tension: 40.4 dyne/cm; (18)Density: 1.283 g/cm3; (19)Flash Point: 79 °C; (20)Enthalpy of Vaporization: 42.28 kJ/mol; (21)Boiling Point: 186.5 °C at 760 mmHg; (22)Vapour Pressure: 0.66 mmHg at 25°C.
Preparation of Phenyl chlorocarbonate: It can be prepared by phenol and phosgene. Dissolve phenol in chloroform, and then pass phosgene. Dilute with water after addition of equimolar N,N-Dimethylaniline. Wash the oil layer with diluted hydrochloric acid and water, and then dry with anhydrous calcium chloride. Heat to get rid of chloroform, and collect the distillate in 74-75°C(1.73kPa) after reduced pressure distillation. The distillate is the product you desired. And the yield is 90%.
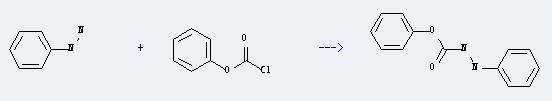
Uses of Phenyl chlorocarbonate: It can react with phenylhydrazine to produce 3-phenyl-carbazic acid phenyl ester. This reaction will need solvents diethyl ether and tetrahydrofuran. The reaction time is 0.5 hours with heating, and the yield is about 32%.
You should be cautious while dealing with this chemical. If contact with water, it will liberate toxic gas. It irritates skin, and has risk of serious damage to eyes. It is very toxic by inhalation, and may also cause burns. In addition, it is harmful if swallowed. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer); In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(Cl)Oc1ccccc1
(2)InChI: InChI=1/C7H5ClO2/c8-7(9)10-6-4-2-1-3-5-6/h1-5H
(3)InChIKey: AHWALFGBDFAJAI-UHFFFAOYAM
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC | inhalation | > 800mg/m3/10M (800mg/m3) | National Defense Research Committee, Office of Scientific Research and Development, Progress Report.Vol. NDCrc-132, Pg. Dec, 1942. | |
| rabbit | LD50 | skin | 3970uL/kg (3.97mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. | |
| rat | LCLo | inhalation | 44ppm/4H (44ppm) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. | |
| rat | LD50 | oral | 1410uL/kg (1.41mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. |
Related Products
- Phenyl (1-piperidinocyclohexyl) ketone
- Phenyl [5-(trifluoromethyl)pyridin-2-yl]carbamate
- Phenyl 1,4-dihydroxy-2-naphthoate
- Phenyl 1-hydroxy-2-naphthoate
- Phenyl 2-bromo-2-propyl ketone
- Phenyl 2-chloroacetate
- Phenyl 2-pyridyl ketoxime
- phenyl 4,5-dimethoxy-2-nitrobenzoate
- Phenyl 4,6-o-benzylidene-1-thio-beta-d-glucopyranoside
- Phenyl 4,6-O-benzylidene-beta-D-glucopyranoside
- 188527-21-1
- 188527-56-2
- 1885-29-6
- 188530-20-3
- 18853-32-2
- 188534-09-0
- 1885-35-4
- 1885-38-7
- 18854-48-3
- 188548-57-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View