-
Name
1,4-Diazacyclohexane
- EINECS 203-808-3
- CAS No. 110-85-0
- Article Data308
- CAS DataBase
- Density 0.874 g/cm3
- Solubility Soluble in water, ethanol. Insoluble in diethyl ether
- Melting Point 109-112 °C(lit.)
- Formula C4H10N2
- Boiling Point 149.324 °C at 760 mmHg
- Molecular Weight 86.1368
- Flash Point 49.726 °C
- Transport Information UN 2579 8/PG 3
- Appearance needle-like white or colorless crystals
- Safety 22-26-36/37/39-45-61
- Risk Codes 34-42/43-52/53
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 1,4-Diazacyclohexane;1,4-Piperazine;Antiren;Diethylenediamine;Dispermine;Eraverm;Hexahydropyrazine;Lumbrical;Pipersol;Pyrazinehexahydride;Uvilon;Vermex;Wurmirazin;Piperazine anhydrous;
- PSA 24.06000
- LogP -0.16320
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; sodium sulfate In 1,4-dioxane at 180℃; under 66756.7 Torr; for 6h; Reagent/catalyst; Autoclave; | 80.7% |
| With aluminum oxide; nitrogen at 400 - 450℃; | |
| With 1,4-dioxane; nickel at 200℃; |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; potassium hydroxide In ethanol at 80℃; under 90009 Torr; Pressure; Reagent/catalyst; Solvent; Temperature; Time; | A 1.03% B 97.53% |

| Conditions | Yield |
|---|---|
| With ammonia; (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In toluene at 155℃; under 42754.3 Torr; for 12h; Autoclave; | |
| With ammonia; (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In toluene at 180℃; under 27002.7 Torr; for 12h; Autoclave; | |
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); ammonia; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] at 155℃; for 12h; Temperature; Inert atmosphere; Autoclave; |
-
-
107-15-3
ethylenediamine

-
-
111-40-0
1,5-diamino-3-azapentane

-
A
-
110-85-0
piperazine

-
B
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

| Conditions | Yield |
|---|---|
| With ZSM-5 type zeolite In water at 370℃; pH=10; Temperature; pH-value; | A 63% B 17% |
| With ZSM-5 type zeolite In water at 330℃; pH=10; Temperature; pH-value; | A 44% B 56% |
| With ZSM-5 type zeolite with Na exchange rate of 62percent In water at 290℃; Reagent/catalyst; Temperature; | |
| With Na-ion exchanged ZSM-5 type zeolite In water at 290 - 600℃; Gas phase; | |
| With β-type iron silicate at 320℃; Reagent/catalyst; |


| Conditions | Yield |
|---|---|
| With hydrogen; sodium sulfate at 210℃; under 88508.9 Torr; for 6h; Reagent/catalyst; Autoclave; | 70% |
| With 1,4-dioxane; hydrogen; nickel at 200℃; under 28 Torr; | |
| With palladium on magnesium oxide In acetonitrile at 160℃; for 6.5h; Autoclave; Inert atmosphere; | 80 %Chromat. |

| Conditions | Yield |
|---|---|
| With ZSM-5 type zeolite In water at 350℃; | A 26% B 53% |
| With water; Na-type ZSM-5(2) at 350 - 370℃; | A 20.2% B 47.7% |
| Na-type ZSM-5(6) at 355 - 380℃; | A 18.9% B 39.9% |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In tetrachloromethane at 30 - 60℃; for 20h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With tetralin; nickel at 150℃; | |
| With tetralin; hydrogen; nickel at 170℃; | |
| With monoaluminum phosphate at 400 - 430℃; |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; ruthenium(1,1,1-tris(di(3,5-dimethylphenyl)phosphinomethyl)ethane)(η4-trimethylenemethane); hydrogen In tetrahydrofuran at 180℃; under 75007.5 Torr; for 16h; Autoclave; Schlenk technique; | 84% |
| With i-Amyl alcohol; sodium | |
| With sulfuric acid | |
| With hydrogenchloride at 20℃; |

| Conditions | Yield |
|---|---|
| With ammonia; C28H44BNOP2Ru In toluene at 180℃; under 21002.1 Torr; for 24h; Product distribution / selectivity; Autoclave; Inert atmosphere; | |
| With ammonia; hydrogen; chlorocarbonylhydrido[4,5-bis(dicyclohexylphosphinomethyl)acridine]ruthenium(II) In toluene at 180℃; under 49580 Torr; for 12h; Product distribution / selectivity; Autoclave; Inert atmosphere; | |
| With ammonia; (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In toluene at 155℃; under 31503.2 Torr; for 12h; Autoclave; |

-
-
7483-59-2
1-(2,3-dihydroxy propyl)piperazine

-
A
-
110-85-0
piperazine

-
B
-
76950-43-1
2-hydroxymethyl-1,4-diazabicyclo<2.2.2>octane

| Conditions | Yield |
|---|---|
| With solid catalyst from aluminum phosphate and cesium nitrate In water at 360℃; for 4h; Inert atmosphere; Large scale; | A 22% B 44% |
| With cesium phosphate and batium hydroxyapatite on Al2O3 In water at 390℃; for 24h; Reagent/catalyst; Temperature; Overall yield = 95 %; |
-
-
75-21-8
oxirane

-
A
-
110-85-0
piperazine

-
B
-
102-71-6
triethanolamine

-
C
-
141-43-5
ethanolamine

-
D
-
107-15-3
ethylenediamine

-
E
-
111-40-0
1,5-diamino-3-azapentane

-
F
-
111-42-2
2,2'-iminobis[ethanol]

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; Ni-Re-B catalyst In water at 165℃; under 156079 Torr; |

-
-
141-43-5
ethanolamine

-
A
-
110-85-0
piperazine

-
B
-
107-15-3
ethylenediamine

-
C
-
111-40-0
1,5-diamino-3-azapentane

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; Nickel/Rhenium/Boron (8.0:2.0: 1.7 wt. percent) catalyst on an alumina/silica (80:20) at 165℃; under 155909 Torr; | |
| With ammonia; hydrogen at 170℃; under 60006 Torr; for 12h; Flow reactor; |


| Conditions | Yield |
|---|---|
| With hydrogen In water at 180℃; under 15001.5 Torr; |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water at 190℃; under 150015 Torr; Temperature; | A 42.5 %Chromat. B 6.3 %Chromat. |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water at 159℃; under 4431.9 Torr; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| With chlorocarbonylhydrido[4,5-bis(dicyclohexylphosphinomethyl)acridine]ruthenium(II); potassium tert-butylate; ammonia In toluene at 180℃; under 35553.6 Torr; for 12h; Autoclave; Inert atmosphere; |
-
-
141-43-5
ethanolamine

-
-
111-42-2
2,2'-iminobis[ethanol]

-
A
-
110-85-0
piperazine

-
B
-
103-76-4
1-(2-hydroxyethyl)piperazine

| Conditions | Yield |
|---|---|
| With hydrogen In 1,4-dioxane at 200℃; under 30003 Torr; Solvent; | |
| With hydrogen In ethanol at 200℃; under 15001.5 Torr; Solvent; Temperature; Pressure; |

-
-
140-31-8
aminoethylpiperazine

-
-
107-15-3
ethylenediamine

-
A
-
110-85-0
piperazine

-
B
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

| Conditions | Yield |
|---|---|
| With ZSM-5 type zeolite In water at 320℃; pH=9; Temperature; pH-value; | A 54% B 46% |


| Conditions | Yield |
|---|---|
| With potassium carbonate; [Ir(cod)(1-neopentyl-4-n-butyl-triazole-5-ylidene)PPh3]BF4 In isopropyl alcohol at 82℃; for 24h; | 100% |
| With dichloro(μ-chloro)(μ-hydrido)bis(η-p-cymene)diruthenium(II); hydrogen In 1,4-dioxane at 75℃; under 37503.8 Torr; for 40h; | 87% |
| With Cp*Rh(2-(2-pyridyl)phenyl)H; hydrogen In neat (no solvent) at 100℃; under 27361.8 Torr; for 48h; Catalytic behavior; Glovebox; | 31% |
-
-
23147-58-2, 110822-84-9, 110822-85-0
glycolaldehyde dimer

-
A
-
110-85-0
piperazine

-
B
-
141-43-5
ethanolamine

-
C
-
107-15-3
ethylenediamine

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In tetrahydrofuran at 100℃; under 75007.5 Torr; for 8h; Autoclave; |

-
-
111-42-2
2,2'-iminobis[ethanol]

-
A
-
110-85-0
piperazine

-
B
-
140-31-8
aminoethylpiperazine

-
C
-
107-15-3
ethylenediamine

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water at 196℃; under 150015 Torr; Temperature; | A 36.7 %Chromat. B 6.7 %Chromat. C 5.1 %Chromat. |

-
-
141-43-5
ethanolamine

-
A
-
110-85-0
piperazine

-
B
-
111-41-1
2-(2-Aminoethylamino)ethanol

-
C
-
107-15-3
ethylenediamine

-
D
-
111-40-0
1,5-diamino-3-azapentane

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen | |
| With ammonia; hydrogen | |
| With ammonia; hydrogen at 170℃; under 60006 Torr; for 12h; Reagent/catalyst; Flow reactor; | |
| With ammonia at 170℃; under 60006 Torr; |

-
-
10430-90-7
piperazine-1-carboxylic acid

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
110-85-0
piperazine

-
B
-
108-32-7
1,2-propylene cyclic carbonate

| Conditions | Yield |
|---|---|
| With potassium iodide at 160℃; for 10h; Reagent/catalyst; | A 97.6% B 95.3% |

-
-
75-21-8
oxirane

-
-
10430-90-7
piperazine-1-carboxylic acid

-
A
-
110-85-0
piperazine

-
B
-
96-49-1
[1,3]-dioxolan-2-one

| Conditions | Yield |
|---|---|
| With zinc dibromide at 160℃; for 8.5h; | A 97.7% B 94.9% |

-
-
17046-84-3
1,4-bis-(toluene-4-sulfonyl)-piperazine

-
-
110-85-0
piperazine

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-bis-(toluene-4-sulfonyl)-piperazine With Na/K absorbed into silica gel In 1,2-dimethoxyethane at 60℃; Inert atmosphere; Stage #2: With water In 1,2-dimethoxyethane | 76% |
| With sulfuric acid at 170℃; |
-
-
107-06-2
1,2-dichloro-ethane

-
A
-
110-85-0
piperazine

-
B
-
112-57-2
N-(2-aminoethyl)-N'-{2-[(2-aminoethyl)amino]ethyl}ethane-1,2-diamine

-
C
-
111-40-0
1,5-diamino-3-azapentane

-
D
-
112-24-3
triethylentetramine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; ethylenediamine In water at 100℃; under 18751.9 Torr; |

-
-
141-43-5
ethanolamine

-
A
-
110-85-0
piperazine

-
B
-
140-31-8
aminoethylpiperazine

-
C
-
107-15-3
ethylenediamine

-
D
-
111-40-0
1,5-diamino-3-azapentane

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water at 80℃; under 26463 Torr; Temperature; Pressure; |

-
-
141-43-5
ethanolamine

-
A
-
110-85-0
piperazine

-
B
-
103-76-4
1-(2-hydroxyethyl)piperazine

-
C
-
107-15-3
ethylenediamine

-
D
-
111-40-0
1,5-diamino-3-azapentane

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water at 103℃; under 26463 Torr; Temperature; |


| Conditions | Yield |
|---|---|
| With 1-(1-methylethyl)piperazine; palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In 1,2-dimethoxyethane Inert atmosphere; | 74% |

| Conditions | Yield |
|---|---|
| In ethylene glycol at 140℃; for 2h; Product distribution / selectivity; | 100% |
| In ethylene glycol at 140℃; for 2h; | 100% |
| With potassium hydroxide In toluene for 4h; Heating; | 83% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; sodium nitrite In dichloromethane at 20℃; chemoselective reaction; | 100% |
| With sulfuric acid; silica gel; sodium nitrite In dichloromethane at 20℃; for 0.166667h; | 99% |
| With cross-linked polyvinylpyrrolidone*N2O4; dinitrogen tetraoxide In dichloromethane at 20℃; for 0.333333h; | 98% |
-
-
110-85-0
piperazine

-
-
72553-83-4
1-Chlor-4-phenyl-pyridazino<4,5-d>pyridazin

-
-
83490-52-2
1-Piperazino-4-phenyl-pyridazino<4,5-d>pyridazin

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 100% |
-
-
110-85-0
piperazine

-
-
626-48-2
6-Methyluracil

-
-
50-00-0
formaldehyd

-
-
75682-13-2
C-5,N-3-Dipiperazinomethylene-6-methyluracil

| Conditions | Yield |
|---|---|
| In ethanol; water for 24h; Ambient temperature; | 100% |

-
-
110-85-0
piperazine

-
-
1635-61-6
5-chloro-2-nitroaniline

-
-
96103-52-5
2-nitro-5-piperazin-1-yl-phenylamine

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 120℃; for 24h; | 100% |
| for 5h; Heating; | 93% |
| With potassium carbonate In N,N-dimethyl acetamide at 120℃; for 21h; | 84% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Reflux; | 100% |
| With triethylamine In ethanol for 21h; Heating; | 45% |
-
-
110-85-0
piperazine

-
-
121-17-5
2-chloro-3-nitro-5-trifluoromethylbenzene

-
-
137918-81-1
1,4-Bis(1'-nitro-3'-(trifluoromethyl)-6'-phenyl)piperazine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 100℃; under 6000480 Torr; for 120h; | 100% |

| Conditions | Yield |
|---|---|
| at 110℃; | 100% |
-
-
110-85-0
piperazine

-
-
122-60-1
Phenyl glycidyl ether

-
-
40944-05-6
1-phenoxy-3-(1-piperazinyl)propan-2-ol

| Conditions | Yield |
|---|---|
| In 2,2,2-trifluoroethanol at 20℃; for 6h; regioselective reaction; | 100% |
| In ethanol for 0.0583333h; Ring cleavage; addition; Irradiation; microwave irradiation; | 84% |
| In methanol at 14.9℃; Rate constant; Thermodynamic data; var. temp., ΔH(excit.), ΔG(excit.), ΔS(excit), E; |
-
-
110-85-0
piperazine

-
-
1027017-16-8
6,7-Difluoro-1-(2-fluoro-phenyl)-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid

| Conditions | Yield |
|---|---|
| In acetonitrile | 100% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 100℃; for 20h; | 100% |
| With triethylamine at 130℃; for 4h; Inert atmosphere; | 98% |
| With potassium carbonate In isopropyl alcohol for 36h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen; chloro(1,5-cyclooctadiene)rhodium(I) dimer In 1,4-dioxane at 80℃; under 82506.6 Torr; for 72h; | 100% |

-
-
110-85-0
piperazine

-
-
10199-89-0
NBD chloride

-
-
139332-66-4
4-nitro-7-(piperazin-1-yl)benzo[c][1,2,5]oxadiazole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; | 100% |
| at 0℃; for 0.833333h; | 95% |
| In N,N-dimethyl-formamide at 85℃; for 4h; Inert atmosphere; Schlenk technique; | 78% |
-
-
110-85-0
piperazine

-
-
207857-15-6
1,3-di(tert-butyloxycarbonyl)-2-(trifluoromethylsulfonyl)guanidine

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 20h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: piperazine With n-butyllithium In tetrahydrofuran at 20℃; for 1h; Metallation; Stage #2: benzoyl chloride In tetrahydrofuran for 0.166667h; Acylation; Stage #3: 2-Methoxybenzoyl chloride In tetrahydrofuran for 0.166667h; Acylation; | 100% |


| Conditions | Yield |
|---|---|
| In ethanol; water for 1h; Heating; | 100% |


| Conditions | Yield |
|---|---|
| In ethanol; water for 1.08333h; Heating; | 100% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 50 - 70℃; for 1.16667h; | 100% |


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In xylene for 4h; Buchwald coupling; Heating; | 100% |
| With dichlorobis(tri-O-tolylphosphine)palladium; potassium tert-butylate In diethylene glycol dimethyl ether at 170℃; for 48h; | 61% |
| With dichlorobis(tri-O-tolylphosphine)palladium; sodium t-butanolate In various solvent(s) at 169℃; for 24h; | 32% |
-
-
110-85-0
piperazine

-
-
33371-00-5
4-chloro-6,7,8-trimethoxy-quinazoline

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |
| Heating; |
-
-
110-85-0
piperazine

-
-
155960-96-6
4-chloro-6,8-dimethoxyquinazoline

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |
| Heating; |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |
| With triethylamine at 80 - 140℃; for 7h; | 78% |
| With triethylamine at 80 - 130℃; Neat (no solvent); | 68% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Heating; | 100% |
| In ethanol at 20 - 60℃; | 61% |
| In ethanol at 60℃; for 5h; | 61% |
Piperazine Consensus Reports
Reported in EPA TSCA Inventory.
Piperazine Standards and Recommendations
DOT Classification: 8; Label: Corrosive
Piperazine Specification
The Piperazine, with the CAS registry number 110-85-0, is also known as 1,4-Diazacyclohexane. It belongs to the product categories of Blocks; BuildingBlocks; Organics; Heterocycles; Isotope Labelled Compounds. Its EINECS number is 203-808-3. This chemical's molecular formula is C4H10N2 and molecular weight is 86.13. What's more, its systematic name is Piperazine. Its classification codes are: (1) Agricultural Chemical; (2)Anthelmintic; (3)Anthelmintics; (4)Anti-Infective Agents; (5)Antinematodal agents; (6)Antiparasitic Agents; (7)Drug / Therapeutic Agent; (8)Human Data; (9)Skin / Eye Irritant; (10)Unspecified / Unclassified pesticide. This chemical should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides, heat and fire. This chemical is commonly used as a pharmaceutical intermediate. It is also used in the manufacture of plastics, resins, pesticides, brake fluid and other industrial materials.
Physical properties of Piperazine are: (1)ACD/LogP: -1.171; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -4.41; (4)ACD/LogD (pH 7.4): -3.28; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 24.06 Å2; (13)Index of Refraction: 1.424; (14)Molar Refractivity: 25.143 cm3; (15)Molar Volume: 98.521 cm3; (16)Polarizability: 9.968×10-24cm3; (17)Surface Tension: 27.6 dyne/cm; (18)Density: 0.874 g/cm3; (19)Flash Point: 49.726 °C; (20)Enthalpy of Vaporization: 38.624 kJ/mol; (21)Boiling Point: 149.324 °C at 760 mmHg; (22)Vapour Pressure: 4.0 mmHg at 25°C.
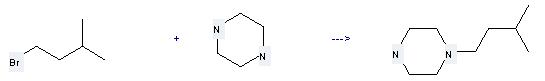
Uses of Piperazine: it can be used to produce 1-(3-methyl-butyl)-piperazine by heating. It will need reagent triethylamine and solvent ethanol with the reaction time of 17 hours. The yield is about 62%.
When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. It may cause sensitisation by inhalation and skin contact. This substance is harmful to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. You should not breathe dust. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). You should avoid releasing it to the environment, and you need refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: C1CNCCN1
(2)Std. InChI: InChI=1S/C4H10N2/c1-2-6-4-3-5-1/h5-6H,1-4H2
(3)Std. InChIKey: GLUUGHFHXGJENI-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 75mg/kg (75mg/kg) | BEHAVIORAL: SLEEP GASTROINTESTINAL: NAUSEA OR VOMITING | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 478, 1969. |
| mouse | LC50 | inhalation | 5400mg/m3/2H (5400mg/m3) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: EXCITEMENT | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 15, Pg. 116, 1979. |
| mouse | LD50 | intraperitoneal | 1900mg/kg (1900mg/kg) | Progress in Biochemical Pharmacology. Vol. 1, Pg. 542, 1965. | |
| mouse | LD50 | intravenous | 1180mg/kg (1180mg/kg) | Drugs in Japan Vol. 6, Pg. 635, 1982. | |
| mouse | LD50 | oral | 600mg/kg (600mg/kg) | Bollettino Chimico Farmaceutico. Vol. 103, Pg. 414, 1964. | |
| rabbit | LD50 | skin | 4mL/kg (4mL/kg) | Union Carbide Data Sheet. Vol. 7/16/1965, | |
| rat | LD50 | intramuscular | > 2500mg/kg (2500mg/kg) | Drugs in Japan Vol. 6, Pg. 635, 1982. | |
| rat | LD50 | intravenous | 1340mg/kg (1340mg/kg) | Drugs in Japan Vol. 6, Pg. 635, 1982. | |
| rat | LD50 | oral | 1900mg/kg (1900mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: EXCITEMENT | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 15, Pg. 116, 1979. |
| rat | LD50 | subcutaneous | 3700mg/kg (3700mg/kg) | Drugs in Japan Vol. 6, Pg. 635, 1982. |
Related Products
- Piperazine
- Piperazine 2-oxobornane-10-sulphonate
- Piperazine Adipate
- Piperazine citrate
- Piperazine diantimony tartrate
- Piperazine dihydrochloride
- Piperazine ferulate
- Piperazine hydrogen phosphate monohydrate
- Piperazine phosphate
- Piperazine sultosilate
- 110859-47-7
- 110859-69-3
- 110860-92-9
- 110-86-1
- 110861-14-8
- 110863-24-6
- 1108668-13-8
- 1108-68-5
- 110871-86-8
- 110-87-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View