-
Name
N-ETHYLPYRROLIDINE
- EINECS 230-840-5
- CAS No. 7335-06-0
- Article Data30
- CAS DataBase
- Density 0.845 g/cm3
- Solubility
- Melting Point 102-104 °C
- Formula C6H13N
- Boiling Point 104 °C at 760 mmHg
- Molecular Weight 99.1759
- Flash Point 1.6 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 10-34
-
Molecular Structure
- Hazard Symbols F,C
- Synonyms N-ethyl-Tetrahydropyrrole;N-Ethylpyrrolidine;N-Ethyltetrahydropyrrole;
- PSA 3.24000
- LogP 1.04000
Synthetic route

| Conditions | Yield |
|---|---|
| With Ru on activatedcarbon cloth; activatedcarbon cloth In aq. phosphate buffer at 60℃; for 10h; pH=8.5; Time; Electrochemical reaction; | 99% |
| rhodium hydrido (PEt3)3 complex for 6h; | 74 % Chromat. |
-
-
72332-01-5
Phenothiazine-10-carboxylic acid 4-diethylamino-butyl ester

-
A
-
7335-06-0
1-ethylpyrrolidine

-
B
-
92-84-2
10H-phenothiazine

| Conditions | Yield |
|---|---|
| at 200℃; for 0.5h; Yields of byproduct given; | A n/a B 89% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 96h; | 80% |
| In methanol at 20℃; |

| Conditions | Yield |
|---|---|
| With hydrogen; RhMoZn/C In di-isopropyl ether at 70 - 160℃; under 7500.75 Torr; for 16h; Conversion of starting material; | 77% |
| With hydrogen; PtReIn/silica In di-isopropyl ether at 70 - 160℃; under 7500.75 Torr; for 16h; Conversion of starting material; | 50% |
| With hydrogen; PtReCu/silica In di-isopropyl ether at 70 - 160℃; under 7500.75 Torr; for 16h; Conversion of starting material; | 40% |

| Conditions | Yield |
|---|---|
| With aluminium oxide thorium oxide-catalyst at 300℃; | |
| With aluminum oxide at 400℃; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
120-94-5
1-Methylpyrrolidine

-
A
-
7335-06-0
1-ethylpyrrolidine

-
B
-
765-48-0, 802254-03-1, 802822-49-7
1,2-dimethylpyrrolidine

-
C
-
45470-22-2
N-methyl-3-methylpyrrolidine

| Conditions | Yield |
|---|---|
| UV-Licht; |

-
-
120-94-5
1-Methylpyrrolidine

-
-
2465-56-7
methylene

-
A
-
7335-06-0
1-ethylpyrrolidine

-
B
-
765-48-0, 802254-03-1, 802822-49-7
1,2-dimethylpyrrolidine

-
C
-
45470-22-2
N-methyl-3-methylpyrrolidine


| Conditions | Yield |
|---|---|
| With palladium at 160℃; Hydrogenation; | |
| With acetic acid; platinum Hydrogenation; |

| Conditions | Yield |
|---|---|
| With sulfuric acid Reduktion unter Verwendung einer Zink-Amalgam-Kathode; |
-
-
16751-58-9
3-aminohexane

-
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| Chlorierung; folgende Behandlung mit Schwefelsaeure; |

| Conditions | Yield |
|---|---|
| With aluminum oxide; nitrogen at 280℃; | |
| Multi-step reaction with 2 steps 1: 1) NaH / 1) toluene, heating, 3 h; 2) heating, 3 h 2: 0.5 h / 200 °C View Scheme |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride |

| Conditions | Yield |
|---|---|
| With ethanol |

| Conditions | Yield |
|---|---|
| With aluminium oxide thorium oxide-catalyst at 300℃; |
-
-
4186-68-9
1-Ethyl-1-methylpyrrolidinium iodide

-
-
141-43-5
ethanolamine

-
A
-
120-94-5
1-Methylpyrrolidine

-
B
-
7335-06-0
1-ethylpyrrolidine

-
C
-
2955-88-6
1-pyrrolidineethanol

-
D
-
624-78-2
N-Ethylmethylamine

-
-
110-63-4
Butane-1,4-diol

-
-
75-04-7
ethylamine

-
A
-
109-99-9
tetrahydrofuran

-
B
-
7335-06-0
1-ethylpyrrolidine

-
C
-
39216-86-9
4-(ethylamino)butan-1-ol

| Conditions | Yield |
|---|---|
| CrZMS-5 at 300℃; for 3h; | A n/a B 59.6 % Chromat. C n/a |


| Conditions | Yield |
|---|---|
| at 30℃; elektrochemischen Reduktoin an einer Zink-Amalgam-Kathode; |

-
-
123-91-1
1,4-dioxane

-
-
4277-64-9
ethyl 1H-pyrrole-1-carboxylate

-
A
-
120-94-5
1-Methylpyrrolidine

-
B
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| at 230℃; under 147102 - 220652 Torr; Hydrogenation; |

-
-
4277-64-9
ethyl 1H-pyrrole-1-carboxylate

-
-
64-17-5
ethanol

-
A
-
120-94-5
1-Methylpyrrolidine

-
B
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| at 230℃; under 147102 - 220652 Torr; Hydrogenation; |

-
-
2314-78-5
N-ethylsuccinimide

-
-
7664-93-9
sulfuric acid

-
-
7732-18-5
water

-
A
-
7335-06-0
1-ethylpyrrolidine

-
B
-
2687-91-4
1-ethyl-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| at 28℃; elektrochemischen Reduktion an einer Blei-Kathode; |


| Conditions | Yield |
|---|---|
| at 400℃; |

-
-
109-99-9
tetrahydrofuran

-
-
7664-41-7
ammonia

-
-
75-04-7
ethylamine

-
A
-
123-75-1
pyrrolidine

-
B
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| at 400℃; |

-
-
123-91-1
1,4-dioxane

-
-
64-17-5
ethanol

-
-
854852-25-8
3-hydroxy-4-nitro-butyric acid ethyl ester

-
A
-
7335-06-0
1-ethylpyrrolidine

-
B
-
2687-91-4
1-ethyl-2-pyrrolidinone

| Conditions | Yield |
|---|---|
| at 260℃; under 191232 Torr; Hydrogenation; |

-
-
110-61-2
butanedinitrile

-
-
75-04-7
ethylamine

-
A
-
7335-06-0
1-ethylpyrrolidine

-
B
-
617-92-5
1-ethyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With hydrogen; palladium |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen at 290℃; under 30003 Torr; for 4h; Autoclave; |
-
-
13360-63-9
N-ethylbutylamine

-
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| With C31H37ClN3NiO2(1-)*Li(1+); potassium tert-butylate In dimethyl sulfoxide at 110℃; for 3h; Catalytic behavior; Sealed tube; Inert atmosphere; | 20.8 %Chromat. |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
98-68-0
4-methoxy-phenyl-sulphonyl chloride

-
-
335215-12-8
1-((4-methoxyphenyl)sulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 98% |
-
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1.5h; | 96% |
-
-
7335-06-0
1-ethylpyrrolidine

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-phenylenebis(propane-3,1-diyl) bis(4-methylbenzenesulfonate) In acetonitrile for 0.0833333h; Inert atmosphere; Stage #2: 1-ethylpyrrolidine In acetonitrile at 20℃; for 1h; Inert atmosphere; | 96% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
16133-25-8
Pyridine-3-sulfonyl chloride

-
-
26103-51-5
3-(pyrrolidin-1-ylsulfonyl)pyridine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 95% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
93-11-8
2-Naphthalenesulfonyl chloride

-
-
111659-88-2
1-(naphthalen-2-ylsulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| In dichloromethane; toluene at 20℃; | 93% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
16629-19-9
thiophene-2-sulfonyl chloride

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With sodium sulfate In water; acetonitrile at 20℃; for 9h; Electrolysis; | 92% |
| With tert.-butylhydroperoxide; iodine In water at 80℃; for 8h; Sealed tube; | 89% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| In dichloromethane; toluene at 20℃; | 91% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
49584-26-1
p-cyanobenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 91% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
15084-51-2
4-tert-butylbenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 91% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
15898-42-7
sodium 4-cyanobenzenesulfinate

| Conditions | Yield |
|---|---|
| With sodium sulfate In water; acetonitrile at 20℃; for 9h; Electrolysis; | 91% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
98-09-9
benzenesulfonyl chloride

-
-
5033-22-7
1-(benzenesulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Catalytic behavior; Reagent/catalyst; Solvent; Sealed tube; chemoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 90% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
873-55-2
sodium benzenesulfonate

-
-
5033-22-7
1-(benzenesulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With sodium sulfate In water; acetonitrile at 20℃; for 9h; Electrolysis; | 90% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 88% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 88% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| In acetone for 2h; Heating; | 85.5% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
349-88-2
4-Fluorobenzenesulfonyl chloride

-
-
157187-14-9
1-((4-fluorophenyl)sulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 85% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
1950-68-1
4-methoxybenzenesulfonyl hydrazide

-
-
335215-12-8
1-((4-methoxyphenyl)sulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In water at 80℃; for 8h; Green chemistry; | 85% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
824-80-6
sodium 4-fluorobenzenesulfinate

-
-
157187-14-9
1-((4-fluorophenyl)sulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With sodium sulfate In water; acetonitrile at 20℃; for 9h; Electrolysis; | 85% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
132481-85-7
3-chloro-4-(trifluoromethyl)benzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 83% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
10151-46-9
2-naphthylsulfonyl hydrazide

-
-
111659-88-2
1-(naphthalen-2-ylsulfonyl)pyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In water at 80℃; for 8h; Green chemistry; | 83% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
-
6435-78-5
N-tosylpyrrolidine

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In water at 80℃; for 8h; Green chemistry; | 82% |
-
-
7335-06-0
1-ethylpyrrolidine

-
-
65604-75-3
4-(tert-butyl)benzenesulfonyl hydrazide

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine In water at 80℃; for 8h; Green chemistry; | 82% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In tetrahydrofuran; decane at 80℃; for 4h; Sealed tube; chemoselective reaction; | 81% |
Pyrrolidine, 1-ethyl- Specification
The Pyrrolidine, 1-ethyl-, with the CAS registry number 7335-06-0, is also known as N-Ethyl-tetrahydropyrrole. Its EINECS number is 230-840-5. This chemical's molecular formula is C6H13N and molecular weight is 99.17. What's more, its systematic name is 1-Ethylpyrrolidine.
Physical properties of Pyrrolidine, 1-ethyl- are: (1)ACD/LogP: 1.29; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.8; (4)ACD/LogD (pH 7.4): -1.57; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.446; (14)Molar Refractivity: 31.34 cm3; (15)Molar Volume: 117.3 cm3; (16)Polarizability: 12.42×10-24cm3; (17)Surface Tension: 26.8 dyne/cm; (18)Density: 0.845 g/cm3; (19)Flash Point: 1.6 °C; (20)Enthalpy of Vaporization: 34.31 kJ/mol; (21)Boiling Point: 104 °C at 760 mmHg; (22)Vapour Pressure: 31.4 mmHg at 25°C.
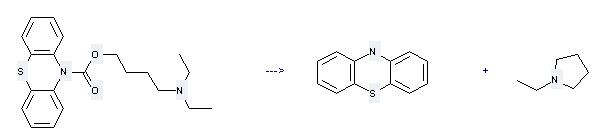
Preparation: this chemical can be prepared by phenothiazine-10-carboxylic acid 4-diethylamino-butyl ester at the temperature of 200°C. This reaction will need the reaction time of 30 min. The yield is about 89%.
Uses of Pyrrolidine, 1-ethyl-: it can be used to produce 2-chloro-4-[N-(4-chlorobutyl)-N-ethylamino]-quinazoline when it is heated. It will need reagent acetone with the reaction time of 2 hours. The yield is about 85.5%.
![Pyrrolidine, 1-ethyl- can be used to produce 2-chloro-4-[N-(4-chlorobutyl)-N-ethylamino]-quinazoline](/UserFilesUpload/Uses of Pyrrolidine, 1-ethyl-.jpg)
When you are using this chemical, please be cautious about it as the following:
It is flammable and easy to cause burn. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: N1(CC)CCCC1
(2)Std. InChI: InChI=1S/C6H13N/c1-2-7-5-3-4-6-7/h2-6H2,1H3
(3)Std. InChIKey: ONQBOTKLCMXPOF-UHFFFAOYSA-N
Related Products
- Pyrrolidine, 1-((1,2,4-benzotriazin-3-yl)acetyl)-
- Pyrrolidine, 1-(1-(3-(p-fluorobenzoyl)propyl)-4-m-tolylisonipecotoyl)-
- Pyrrolidine, 1-(2-phenylcyclohexyl)-, hydrochloride, cis-
- Pyrrolidine, 1-(methyl-d<sub>3</sub>)- (9CI)
- Pyrrolidine, 1-(p-((p-ethylphenyl)azo)phenyl)-
- Pyrrolidine, 1,1'-[(phenylsulfinyl)phosphinylidene]bis- (9CI)
- Pyrrolidine, 1-ethyl-
- Pyrrolidine, 1-hexadecyl-, 1-oxide
- Pyrrolidine, 2-(2,3-dichlorophenyl)-
- Pyrrolidine, 2-(2,3-dimethoxyphenyl)-
- 7335-17-3
- 7335-25-3
- 7335-26-4
- 7335-27-5
- 73354-15-1
- 73355-43-8
- 7335-65-1
- 73357-18-3
- 73358-82-4
- 73360-54-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View