-
Name
Chloroacetic acid sodium salt
- EINECS 223-498-3
- CAS No. 3926-62-3
- Article Data16
- CAS DataBase
- Density 1.399 g/cm3
- Solubility 440 g/L (20 °C) in water
- Melting Point 199 °C (dec.)(lit.)
- Formula C2H3ClO2Na
- Boiling Point 189 °C at 760 mmHg
- Molecular Weight 116.479
- Flash Point 71.5 °C
- Transport Information UN 2659 6.1/PG 3
- Appearance white crystalline powder
- Safety 22-37-45-61
- Risk Codes 25-38-50
-
Molecular Structure
-
Hazard Symbols
 T,
T,  N
N
- Synonyms Aceticacid, chloro-, sodium salt (8CI,9CI);Chloroacetic acid sodium salt;Monochloroacetic acid sodium salt;SMA;SMA (herbicide);Sodium2-chloroacetate;Sodiummonochloroacetate;Sodium a-chloroacetate;
- PSA 40.13000
- LogP -1.02490
Synthetic route

| Conditions | Yield |
|---|---|
| With KB-4P-2(Na) Equilibrium constant; exchange equilibrium constant; | |
| With sodium carbonate In water at 45℃; | |
| With sodium carbonate In water |

| Conditions | Yield |
|---|---|
| In water |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
832151-92-5
(R)-1-chloro-3-(tert-butyldimethylsilyloxy)butan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: sodium monochloroacetic acid; (R)-ethyl 2-((tert-butyldimethylsilyl)oxy)propanoate With tert-butylmagnesium chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 25℃; for 2h; pH=6.0; | 100% |

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| With tert-butylmagnesium chloride; triethylamine In tetrahydrofuran at -5 - 10℃; Inert atmosphere; | 100% |
-
-
53008-65-4
methyl 2,3,4-tri-O-benzyl-D-glucopyranoside

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2,3,4-tri-O-benzyl-D-glucopyranoside With sodium hydride In N,N-dimethyl-formamide at 0℃; Stage #2: sodium monochloroacetic acid at 0 - 20℃; for 0.166667h; | 100% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 78℃; for 24h; Inert atmosphere; | 99% |
-
-
105198-38-7, 106513-42-2
ethyl (S)-2-(tert-butyldimethylsilyloxy)propanoate

-
-
3926-62-3
sodium monochloroacetic acid

-
-
156093-90-2
(S)-3-[(tert-butyldimethyl)silyloxy]-1-chlorobutan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: ethyl (S)-2-(tert-butyldimethylsilyloxy)propanoate; sodium monochloroacetic acid With tert-butylmagnesium chloride; triethylamine In tetrahydrofuran at 0℃; for 4h; Stage #2: With hydrogenchloride In tetrahydrofuran; water pH=6.0; | 98% |
-
-
96-29-7
ethyl methyl ketone oxime

-
-
3926-62-3
sodium monochloroacetic acid

-
-
98548-96-0
2-[(butan-2-ylideneamino)oxy]ethanoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water; acetone at 20 - 60℃; for 2.25h; Solvent; Temperature; | 98% |

| Conditions | Yield |
|---|---|
| In ethanol; diethylperfluoroethylamine at 70 - 78℃; for 4h; | 97.5% |
-
-
216659-47-1
(3-dimethylamino-propyl)-carbamic acid 1,1-dimethylethyl ester

-
-
3926-62-3
sodium monochloroacetic acid

-
-
918106-95-3
C12H24N2O4

| Conditions | Yield |
|---|---|
| In water; tert-butyl alcohol at 80℃; for 8h; | 97.3% |
-
-
68388-21-6
12,13-tetracosanediol

-
-
3926-62-3
sodium monochloroacetic acid

-
-
124029-05-6
(2-Carboxymethoxy-1-undecyl-tridecyloxy)-acetic acid

| Conditions | Yield |
|---|---|
| With benzyltrimethylammonium chloroacetate; potassium tert-butylate In tetrahydrofuran for 30h; Heating; | 97% |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
124029-03-4
1-(2-Hydroxy-tetradecyloxy)-tetradecan-2-ol

-
-
124029-04-5
[1-(2-Carboxymethoxy-tetradecyloxymethyl)-tridecyloxy]-acetic acid

| Conditions | Yield |
|---|---|
| With benzyltrimethylammonium chloroacetate; potassium tert-butylate In tetrahydrofuran for 30h; Heating; | 97% |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
867202-85-5
(2-iodo-benzylsulfanyl)-acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-Iodobenzyl bromide With thiourea In water at 60℃; for 0.5h; Heating / reflux; Stage #2: With sodium hydroxide In water at 60℃; for 0.0833333h; Heating / reflux; Stage #3: sodium monochloroacetic acid In water at 110℃; for 1h; | 97% |
-
-
51698-64-7
2-propylcarbonothioylthiomethylbenzene

-
-
3926-62-3
sodium monochloroacetic acid

-
-
75-04-7
ethylamine

-
A
-
119812-11-2
sodium benzylsulfanylacetate

-
B
-
141-98-0
O-isopropyl-N-ethylthionocarbamate

| Conditions | Yield |
|---|---|
| Stage #1: 2-propylcarbonothioylthiomethylbenzene; ethylamine In water at 70℃; for 1h; Stage #2: sodium monochloroacetic acid In water at 70℃; for 2h; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 70 - 75℃; | 96.1% |
-
-
39669-97-1
N-hexadecanoyl-N',N'-dimethyl-1,3-diaminopropane

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 80℃; for 6h; pH=7 - 8; Temperature; | 95.32% |

| Conditions | Yield |
|---|---|
| Stage #1: sodium monochloroacetic acid; sodium phenoxide In water at 90℃; for 2h; Stage #2: With hydrogenchloride In water at 30℃; pH=0; | 95% |

| Conditions | Yield |
|---|---|
| 95% |

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 3h; Heating; | 95% |
-
-
1282-43-5
(h(5)-methylcyclopentadienyl)(h(5)-cyclopentadienyl)titanium dichloride

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: NaCl; refluxed for 8-10 h; cooled, filtered, concd. in vacuo, petroleum ether (60-80°C) added dropwise with stirring, pptn. collected, dried in vacuo, recrystd. from CHCl3, elem. anal.; | 95% |
| With HCl In water byproducts: NaCl; Ti-compd. refluxed in water for 30 min (pH adjusted to 1.2 by HCl), aq.soln. of ClCH2COONa added dropwise with stirring; pptn. extd. in CH2Cl2, combined extract dried over CaCl2, concd. in vacuo, petroleum ether (60-80°C) added dropwise with stirring, pptn.collected, dried in vacuo, recrystd. from CHCl3; | 90% |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
2344-70-9, 14786-43-7, 22148-86-3, 39516-03-5
(R)-4-phenyl-2-butanol

| Conditions | Yield |
|---|---|
| Stage #1: (R)-4-phenyl-2-butanol With sodium hydride In 1,4-dioxane at 20℃; for 2h; Inert atmosphere; Stage #2: sodium monochloroacetic acid In 1,4-dioxane Inert atmosphere; Reflux; | 95% |
-
-
45267-19-4
myristamidopropyl dimethylamine

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 80℃; for 6h; pH=7 - 8; | 94.86% |

| Conditions | Yield |
|---|---|
| In ethanol; water at 78℃; for 24h; Inert atmosphere; | 94.8% |
-
-
43153-76-0
1-(2-mercaptophenyl)-pyrrole

-
-
3926-62-3
sodium monochloroacetic acid

-
-
80008-51-1
1-(2-carbossimetiltiofenil)pirrolo

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 0.5h; Ambient temperature; | 94.7% |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
578-95-0
10H-acridin-9-one

-
-
58880-43-6
10-Carboxymethyl-10H-acridine-9-one sodium salt

| Conditions | Yield |
|---|---|
| With 1-octyl-3-methylimidazole hexafluorophosphate at 35℃; for 8h; Ionic liquid; | 94.1% |
-
-
3926-62-3
sodium monochloroacetic acid

-
-
42926-80-7
5'-O-(4-monomethoxytrityl)-2'-deoxythymidine

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide at 20℃; for 72h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide With ammonium hydroxide In water at 40℃; for 0.5h; Stage #2: sodium monochloroacetic acid In water for 0.416667 - 0.583333h; Stage #3: With hydrogenchloride In water at 100 - 110℃; | 94% |
| Stage #1: carbon disulfide With ammonia Stage #2: sodium monochloroacetic acid at 80℃; |

-
-
3926-62-3
sodium monochloroacetic acid

| Conditions | Yield |
|---|---|
| In acetonitrile excess carboxylate; alternatively used solvent: methanol; free acid was neutralized with stoichiometric LiOH or triethylamine;; elem. anal.;; | 93% |
-
-
1198181-38-2
1-[3-fluoro-5-(trifluoromethyl)phenyl]pyrrolidin-3-ol

-
-
3926-62-3
sodium monochloroacetic acid

-
-
1198180-52-7
({1-[3-fluoro-5-(trifluoromethyl)phenyl]pyrrolidin-3-yl}oxy)acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1-[3-fluoro-5-(trifluoromethyl)phenyl]pyrrolidin-3-ol With sodium hydride In tetrahydrofuran; mineral oil at 60℃; Stage #2: sodium monochloroacetic acid With tetrabutylammomium bromide In tetrahydrofuran; mineral oil at 60℃; for 2h; Stage #3: With hydrogenchloride In water | 93% |
| Stage #1: 1-[3-fluoro-5-(trifluoromethyl)phenyl]pyrrolidin-3-ol With sodium hydride In tetrahydrofuran; oil at 60℃; for 0.5h; Stage #2: sodium monochloroacetic acid With tetrabutylammomium bromide In tetrahydrofuran; oil at 60℃; for 2h; Stage #3: With hydrogenchloride In water | 93% |
-
-
15684-35-2
cobalt(II) tetrafluoroborate hexahydrate

-
-
7732-18-5
water

-
-
80937-33-3
oxygen

-
-
3926-62-3
sodium monochloroacetic acid

-
-
202128-99-2
2,6‑bis{[bis(2‑pyridylmethyl)amino]methyl}‑4‑t‑butylphenol

| Conditions | Yield |
|---|---|
| With air In acetone at 20℃; | 93% |

-
-
3926-62-3
sodium monochloroacetic acid

-
-
79-04-9
chloroacetyl chloride

-
-
541-88-8
Chloroacetic anhydride

| Conditions | Yield |
|---|---|
| In toluene at 40℃; for 3h; Solvent; Temperature; | 93% |
-
-
66654-01-1
N-[3-(dimethylamino)propyl]undec-10-enamide

-
-
3926-62-3
sodium monochloroacetic acid

-
-
133798-12-6
undecylenamidopropyl betaine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water for 8h; Reflux; | 92.76% |
Sodium chloroacetate Chemical Properties
Molecular Structure of Sodium chloroacetate (CAS NO.3926-62-3):
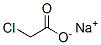
IUPAC Name: sodium 2-chloroacetate
Molecular formula: C2H2ClNaO2
Molecular Weight: 116.48
EINECS: 223-498-3
Merck: 14,2112
BRN: 3597157
Boiling Point: 189 °C at 760 mmHg
Flash Point: 71.5 °C
Enthalpy of Vaporization: 46.87 kJ/mol
Vapour Pressure: 0.259 mmHg at 25°C
Water Solubility: 440 g/L (20°C)
Melting point: 199 °C (dec.)(lit.)
Sensitive: Hygroscopic
InChI
InChI=1/C2H3ClO2.Na/c3-1-2(4)5;/h1H2,(H,4,5);/q;+1/p-1
Smiles
[O-]C(CCl)=O.[Na+]
Stability: Stable. Incompatible with strong oxidizing agents.
Classification Code: Agricultural Chemical; Growth regulator / Fertilizer; Herbicide; Out-dated pesticide
Sodium chloroacetate Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - domestic | LDLo | oral | 100mg/kg (100mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: COMA GASTROINTESTINAL: OTHER CHANGES | Acta Pharmacologica et Toxicologica. Vol. 18, Pg. 179, 1961. |
| chicken | LD50 | oral | 81mg/kg (81mg/kg) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 1047, 1966. | |
| guinea pig | LD50 | intraperitoneal | 115mg/kg (115mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(12), Pg. 53, 1987. | |
| guinea pig | LD50 | oral | 99mg/kg (99mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 23, Pg. 78, 1941. | |
| mammal (species unspecified) | LDLo | oral | 150mg/kg (150mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Nordisk Veterinaermedicin Danske Udgave. Vol. 13, Pg. 271, 1961. |
| mouse | LD50 | oral | 165mg/kg (165mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 86, Pg. 336, 1946. | |
| rabbit | LD50 | oral | 156mg/kg (156mg/kg) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 1047, 1966. | |
| rat | LD50 | oral | 95mg/kg (95mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 23, Pg. 78, 1941. |
Sodium chloroacetate Consensus Reports
Reported in EPA TSCA Inventory.
Sodium chloroacetate Safety Profile
Poison by ingestion and intraperitoneal routes. When heated to decomposition it emits toxic fumes of Cl− and Na2O. Used as an herbicide.
The Hazard Codes:  T,
T,  N
N
HazardClass: 6.1
Risk Statements information:
25: Toxic if swallowed
38: Irritating to the skin
50: Very Toxic to aquatic organisms
Safety Statements information:
22: Do not breathe dust
37: Wear suitable gloves
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
61: Avoid release to the environment. Refer to special instructions safety data sheet
RIDADR: UN 2659 6.1/PG 3
RTECS: AG1400000
PackingGroup: III
HS Code: 29154000
WGK Germany: 2
Sodium chloroacetate Standards and Recommendations
DOT Classification: 6.1; Label: KEEP AWAY FROM FOOD
Sodium chloroacetate Specification
Sodium chloroacetate , with CAS number of 3926-62-3, can be called Caswell No. 755A ; Chloroacetic acid sodium salt ; Chloroctan sodny ; Monoxone ; Sodium monochloroacetate . Sodium chloroacetate (CAS NO.3926-62-3) is used for pesticide, medicine and other organic synthesis.
Related Products
- Sodium
- Sodium 2,4-dimethylbenzenesulfonate
- SODIUM γ-FLUORO-β-HYDROXYBUTYRATE
- Sodium ((3-methoxy-1-methyl-3-oxo-1-propenyl)amino)phenylacetate
- Sodium (+)-10-camphorsulfonate
- Sodium (2-carbamoylphenoxy)acetate
- Sodium (2-methyl-4-chlorophenoxy)acetate
- Sodium (R,R)-5-(2-((2-(3-chlorophenyl)-2-hydroxyethyl)amino)propyl)-1,3-benzodioxole-2,2-
- Sodium (S)-lactate
- Sodium 1,2,3-triazole-5-thiolate
- 39267-04-4
- 39267-05-5
- 3926-71-4
- 39267-79-3
- 39268-71-8
- 39268-74-1
- 39268-96-7
- 39269-10-8
- 39270-39-8
- 39270-55-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View