-
Name
Sulfisoxazole
- EINECS 204-858-9
- CAS No. 127-69-5
- Article Data14
- CAS DataBase
- Density 1.411 g/cm3
- Solubility Water: <0.1 g/100 mL at 22.5 °C
- Melting Point 195 °C
- Formula C11H13N3O3S
- Boiling Point 482.207 °C at 760 mmHg
- Molecular Weight 267.309
- Flash Point 245.43 °C
- Transport Information
- Appearance white to cream powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3,4-Dimethyl-5-sulfanilamidoisoxazole;3,4-Dimethylisoaxazole-5-sulfanilimide;4-Amino-N-(3,4-dimethyl-5-isoxazolyl)benzenesulfonamide;
- PSA 106.60000
- LogP 3.40940
Synthetic route
-
-
184644-22-2
N-(3,4-dimethylisoxazol-5-yl)-4-nitrobenzenesulfonamide

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In acetonitrile at 0 - 20℃; | 69% |
| With hydrogenchloride; zinc | |
| With hydrogenchloride; zinc |
-
-
122447-83-0
2-methyl-N-sulfanilyl-acetoacetamidine

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydroxylamine |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With sodium hydroxide | |
| With sodium hydroxide In methanol Deacetylation; Heating; |
-
-
19947-75-2
5-amino-3,4-dimethylisoxazol

-
-
24939-24-0
4-aminobenzenesulfonyl chloride

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| With pyridine |
-
-
19947-75-2
5-amino-3,4-dimethylisoxazol

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / 20 °C 2: aq. NaOH / methanol / Heating View Scheme | |
| Multi-step reaction with 2 steps 2: aqueous NaOH View Scheme | |
| Multi-step reaction with 2 steps 2: aqueous NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: triethylamine; dmap / dichloromethane / 42 h 2: acetic acid; zinc / acetonitrile / 0 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / 25 °C / Inert atmosphere 2: sodium hydroxide; water / 2 h / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / 20 °C 2: aq. NaOH / methanol / Heating View Scheme |
-
-
174079-07-3
(3,4-Dimethyl-isoxazol-5-yl)-carbamic acid tert-butyl ester

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: TFA / 20 °C 2: pyridine / 20 °C 3: aq. NaOH / methanol / Heating View Scheme |
-
-
857728-49-5
2-methyl-3-oxo-butyrimidic acid ethyl ester

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: NH2OH+HCl View Scheme |
-
-
108846-04-4
N-(N-acetyl-sulfanilyl)-2-methyl-acetoacetamidine

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NH2OH+HCl 2: aqueous NaOH View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: NH2OH+HCl View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine; dmap / dichloromethane / 42 h 2: acetic acid; zinc / acetonitrile / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: aqueous NaOH View Scheme | |
| Multi-step reaction with 2 steps 2: aqueous NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / 25 °C / Inert atmosphere 2: sodium hydroxide; water / 2 h / 100 °C View Scheme |
-
-
4468-47-7, 128252-16-4
2-methyl-3-oxo-butyronitrile

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: Na2CO3; SO2; HSO; NaNO2 3: aqueous NaOH View Scheme | |
| Multi-step reaction with 3 steps 1: Na2CO3; SO2; HSO; NaNO2 3: aqueous NaOH View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogenchloride; hydroxylamine 3: hydrogenchloride; zinc View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogenchloride; hydroxylamine 3: sodium hydroxide View Scheme |
-
-
19947-75-2
5-amino-3,4-dimethylisoxazol

-
-
121-60-8
p-acetylaminobenzenesulfonyl chloride

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: sodium hydroxide / water View Scheme | |
| Multi-step reaction with 2 steps 2: sodium hydroxide View Scheme |
-
-
19947-75-2
5-amino-3,4-dimethylisoxazol

-
-
98-74-8
4-Nitrobenzenesulfonyl chloride

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: hydrogenchloride; zinc View Scheme |

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 92% |
| In ethanol for 4h; Heating; |

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 90% |
| In ethanol for 4h; Heating; |
-
-
127-69-5
sulfisoxazole

-
-
2949-22-6
Glycine ethyl ester isocyanate

-
-
166963-50-4
4-[N '-(Ethoxycarbonylmethyl)ureido]-N-(3,4-dimethyl-5-isoxazolyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 90% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 20℃; for 2h; | 90% |
-
-
346-55-4
4-chloro-7-trifluoromethyl quinoline

-
-
127-69-5
sulfisoxazole

-
-
1489236-22-7
N-(3,4-dimethylisoxazol-5-yl)-4-(7-(trifluoromethyl)quinolin-4-ylamino)benzenesulfonamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 12h; Reflux; | 89% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 6h; Reflux; | 88% |

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 86% |
| In ethanol for 2h; Reflux; | 83% |
| In ethanol for 4h; Heating; |
-
-
33987-02-9
4-chlorobenzoquinazoline

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 24h; Reflux; | 86% |
-
-
127-69-5
sulfisoxazole

-
-
127172-22-9
(E)-3-(N,N-dimethylamino)-1-(3,4-dimethoxyphenyl)-2-propen-1-one

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 22h; Reflux; | 86% |
-
-
127-69-5
sulfisoxazole

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 2h; Microwave irradiation; Reflux; | 85.73% |
-
-
127-69-5
sulfisoxazole

-
-
80756-85-0
(Z)-S-benzo[d]thiazol-2-yl 2-(2-aminothiazol-4-yl)-2-(methoxyimino)ethanethioate

| Conditions | Yield |
|---|---|
| With pyridine; triethylamine In ethanol; dichloromethane at 20℃; Inert atmosphere; | 83.1% |

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 83% |
| In ethanol for 4h; Heating; |

-
-
127-69-5
sulfisoxazole

-
-
1491007-12-5
(E)-N-(4-(N-(3,4-dimethylisoxazol-5-yl)sulfamoyl)phenyl)-N'-(7-(trifluoromethyl)quinolin-4-yl)carbamimidothioic acid

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 24h; Reflux; | 82% |
-
-
127-69-5
sulfisoxazole

-
-
36140-83-7
1-cyanoacetyl-3,5-dimethylpyrazole

-
-
546090-57-7
2-cyano-N-(4-{[(3,4-dimethylisoxa-zole-5-yl)amino]sulfonyl}phenyl)acetamide

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 6h; Reflux; | 82% |
| In 1,4-dioxane for 4h; Reflux; | |
| In 1,4-dioxane |
-
-
127-69-5
sulfisoxazole

-
-
371756-61-5
((E)-3-(3-(dimethylamino)acryloyl))-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| In ethanol for 21h; Reflux; | 81% |
-
-
127-69-5
sulfisoxazole

-
-
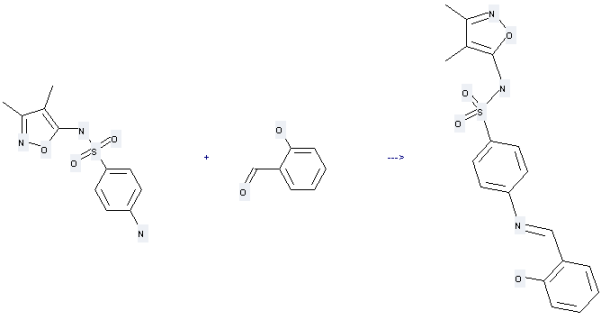
90-02-8
salicylaldehyde

-
-
71308-95-7
(E)-N-(3,4-dimethylisoxazol-5-yl)-4-[(2-hydroxybenzylidene)amino]benzenesulfonamide

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid In ethanol for 4h; Heating; | 80% |
| In ethanol for 4h; Heating; | |
| With acetic acid In ethanol for 4h; Reflux; |
-
-
127-69-5
sulfisoxazole

-
-
708-06-5
2-hydroxynaphthalene-1-carbaldehyde

-
-
84819-63-6
2-hydroxy-1-naphthalidene-sulphafurazole

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 80% |
| In ethanol for 2h; Heating; |
-
-
127-69-5
sulfisoxazole

-
-
93-91-4
1-phenylbutan-1,3-dione

-
-
275801-84-8
N-(3,4-Dimethyl-isoxazol-5-yl)-4-[(Z)-3-hydroxy-1-methyl-3-phenyl-prop-2-en-(E)-ylideneamino]-benzenesulfonamide

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 80% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 18h; Reflux; | 79% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 22h; Reflux; | 77% |

| Conditions | Yield |
|---|---|
| In acetic acid at 90℃; for 3h; | 77% |
-
-
28900-10-9
2-chloro-6-methylnicotinonitrile

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide for 18h; Reflux; | 76% |
-
-
127-69-5
sulfisoxazole

-
-
3173-53-3
Cyclohexyl isocyanate

-
A
-
166963-51-5
4-(N'-Cyclohexylureido)-N-(3,4-dimethyl-5-isoxazolyl)benzene sulfonamide

| Conditions | Yield |
|---|---|
| A n/a B 75% |

-
-
127-69-5
sulfisoxazole

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 4h; Reflux; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride; 5-Bromo-2-aminobenzoic acid for 4h; Reflux; Stage #2: sulfisoxazole With acetic acid for 4h; Reflux; | 75% |

-
-
127-69-5
sulfisoxazole

-
-
36185-83-8
N'-(2-cyanophenyl)-N,N-dimethylimidoformamide

| Conditions | Yield |
|---|---|
| With acetic acid at 100℃; for 3h; | 74% |
-
-
127-69-5
sulfisoxazole

-
-
1761-61-1
5-bromosalicyclaldehyde

-
-
58757-70-3
N4-(5-Bromosalicylidene)-N1-(3,4-dimethyl-5-isoxazolyl)sulfanilamide

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 73% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 24h; Inert atmosphere; Reflux; | 72% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 24h; Inert atmosphere; Reflux; | 72% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 24.25h; Reflux; | 72% |
Sulfisoxazole Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 347.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 ,1980,p. 275.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 24 ,1980,p. 275.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . NCI Carcinogenesis Bioassay (gavage); No Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-138 ,1979. . Reported in EPA TSCA Inventory.
Sulfisoxazole Specification
The Sulfisoxazole, also known as 3,4-Dimethyl-5-sulfanilamidoisoxazole, is sulfonamide antibacterial with the formula C11H13N3O3S. It belongs to the product category of Endothelin Receptor. Its EINECS registry number is 204-858-9. With the CAS registry number 127-69-5, its IUPAC name is 4-amino-N-(3,4-dimethyl-1,2-oxazol-5-yl)benzenesulfonamide. It is sometimes given in combination with erythromycin or phenazopyridine. It is used locally in a 4% solution or ointment.
Physical properties of Sulfisoxazole: (1)ACD/LogP: 1.01; (2)ACD/LogD (pH 5.5): 0.92; (3)ACD/LogD (pH 7.4): -0.22; (4)ACD/BCF (pH 5.5): 2.78; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 68.12; (7)ACD/KOC (pH 7.4): 4.98; (8)#H bond acceptors: 6; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 3; (11)Index of Refraction: 1.626; (12)Molar Refractivity: 67.07 cm3; (13)Molar Volume: 189.4 cm3; (14)Surface Tension: 66.3 dyne/cm; (15)Density: 1.411 g/cm3; (16)Flash Point: 245.4 °C; (17)Enthalpy of Vaporization: 74.71 kJ/mol; (18)Boiling Point: 482.2 °C at 760 mmHg; (19)Vapour Pressure: 1.86E-09 mmHg at 25°C.
Uses of Sulfisoxazole: it can be used to produce N-salicylidene-sulfanilic acid-(3,4-dimethyl-isoxazol-5-ylamide) by heating. This reaction will need reagents anhydrous sodium acetate, glacial acetic acid and solvent ethanol with reaction time of 4 hours. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC1=C(ON=C1C)NS(=O)(=O)C2=CC=C(C=C2)N
(2)InChI: InChI=1S/C11H13N3O3S/c1-7-8(2)13-17-11(7)14-18(15,16)10-5-3-9(12)4-6-10/h3-6,14H,12H2,1-2H3
(3)InChIKey: NHUHCSRWZMLRLA-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | > 5gm/kg (5000mg/kg) | Drugs in Japan Vol. 6, Pg. 391, 1982. | |
| mouse | LD50 | intravenous | 2500mg/kg (2500mg/kg) | Japanese Journal of Toxicology. Vol. 4, Pg. 105, 1991. | |
| mouse | LD50 | oral | 6800mg/kg (6800mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 15, Pg. 1441, 1965. | |
| rabbit | LD50 | oral | 20gm/kg (20000mg/kg) | Drugs in Japan Vol. 6, Pg. 391, 1982. | |
| rat | LD50 | oral | 10gm/kg (10000mg/kg) | Drugs in Japan Vol. 6, Pg. 391, 1982. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View