-
Name
thiosemicarbazide
- EINECS 201-184-7
- CAS No. 79-19-6
- Article Data63
- CAS DataBase
- Density 1.376 g/cm3
- Solubility soluble in water
- Melting Point 180-183 °C (dec.)(lit.)
- Formula CH5N3S
- Boiling Point 208.6 °C at 760 mmHg
- Molecular Weight 91.1368
- Flash Point 80 °C
- Transport Information UN 2811 6.1/PG 2
- Appearance white crystalline solid
- Safety 22-26-36/37-45-61-28A-36/37/39
- Risk Codes 28-52/53-26/27/28
-
Molecular Structure
-
Hazard Symbols
 T+
T+
- Synonyms 1-Aminothiourea;aminothiourea hydrochloride;Isothiosemicarbazide (VAN);Hydrazinecarbothioamide, monohydrochloride;Thiocarbamylhydrazine;1-Amino-2-thiourea;3-Thiosemicarbazide;2-Thiosemicarbazide;Hydrazinecarbothioamide;Semicarbazide, 3-thio-;Semicarbazide, thio-;Semicarbazide, thio-, monohydrochloride;Thiocarbamoylhydrazine;aminothiourea;Aminothio-urea;USAF EK-1275;Isothiosemicarbazide;Thiosemicarbazide;
- PSA 96.16000
- LogP 0.48480
Synthetic route

| Conditions | Yield |
|---|---|
| In ethanol | 95.3% |
-
-
137768-73-1
1-isothiocyanatopropa-1,2-diene

-
A
-
1416132-20-1
1-amino-5-methylimidazole-2(3H)-thione

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In methanol at 0 - 20℃; for 24h; Reagent/catalyst; | A 21% B 77% |

-
-
33186-00-4
6-p-chlorostyryl-3-thioxo-1,2,4-triazin-5-one

-
A
-
33185-98-7
C11H10ClN3O2S

-
B
-
91732-86-4
6-p-chlorostyryl-5-phenyl-1,2,4-triazine-3-thione

-
C
-
91732-87-5
C17H14ClN3OS

-
D
-
91732-89-7
6-p-chlorostyryl-5,5-diphenyl-1,2,4-triazine-3-thione

-
E
-
91732-88-6
C23H20ClN3OS

-
F
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| In diethyl ether; benzene for 5h; Product distribution; Mechanism; Heating; | A 8% B 12% C 5% D 5% E 8% F 10% G n/a |


| Conditions | Yield |
|---|---|
| With ethanol; hydrazine hydrate |

| Conditions | Yield |
|---|---|
| With water; hydrazinium sulfate zuletzt in Aethylenglykol-methylaether oder -aethylaether bei 130grad; | |
| With water; hydrazinium sulfate | |
| With water; potassium carbonate; hydrazinium sulfate Ueber mehrere Stufen; | |
| With hydrazinium sulfate |

| Conditions | Yield |
|---|---|
| With water; hydrazinium sulfate |
-
-
1147550-11-5
ammonium thiocyanate

-
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 130℃; |

| Conditions | Yield |
|---|---|
| With water; hydrazine |
-
-
100-63-0
phenylhydrazine

-
-
108-88-3
toluene

-
-
1752-30-3
Acetone thiosemicarbazone

-
A
-
79-19-6
thiosemicarbazide

-
B
-
103-02-6
acetone phenylhydrazone

| Conditions | Yield |
|---|---|
| at 135℃; |

-
-
2302-93-4
acetophenonethiosemicarbazone

-
-
100-63-0
phenylhydrazine

-
-
108-88-3
toluene

-
A
-
79-19-6
thiosemicarbazide

-
B
-
583-11-9, 59130-82-4
N-(1-phenylethylidene)phenylhydrazine

| Conditions | Yield |
|---|---|
| at 135℃; |


-
-
100-63-0
phenylhydrazine

-
-
108-88-3
toluene

-
A
-
1788-30-3
phenylhydrazone of dibenzyl ketone

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| at 135℃; |


| Conditions | Yield |
|---|---|
| With water at 25 - 50℃; Kinetics; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With water at 25 - 50℃; Kinetics; Equilibrium constant; |
-
-
90049-91-5
Pinakolon-thiosemicarbazon

-
A
-
75-97-8
3,3-dimethyl-butan-2-one

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With water at 25 - 50℃; Kinetics; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With water at 25 - 50℃; Kinetics; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With water at 25 - 50℃; Kinetics; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With water at 25 - 50℃; Kinetics; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With ethylphosphonate buffer at 25℃; Equilibrium constant; |

-
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With water at 130℃; | |
| With xylene at 120℃; |

-
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With sulfuric acid Electrolysis.Bei der elektrochemischen Oxydation an einer Blei-Anode; |
-
-
41763-23-9
1-ethyl-1-phenyl-dithiobiuret

-
A
-
79-19-6
thiosemicarbazide


| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
745076-13-5
C17H17N3OS2

-
A
-
10424-68-7
1,1'-(sulfonylbis(4,1-phenylene))bis(ethan-1-one)

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In water at 20℃; for 168h; |
-
-
745076-31-7
1-[4-(4-{1-[(4-methyl-5H-thiazol-2-ylidene)-hydrazono]-ethyl}-phenylsulfanyl)-phenyl]-ethanone

-
A
-
10424-68-7
1,1'-(sulfonylbis(4,1-phenylene))bis(ethan-1-one)

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid at 20℃; for 168h; |
-
-
745076-16-8
C18H20N6OS2

-
A
-
10424-68-7
1,1'-(sulfonylbis(4,1-phenylene))bis(ethan-1-one)

-
B
-
563-41-7
semicarbazide hydrochloride

-
C
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid at 20℃; for 168h; |

-
-
745076-33-9
C21H22N6S3

-
A
-
10424-68-7
1,1'-(sulfonylbis(4,1-phenylene))bis(ethan-1-one)

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid at 20℃; for 168h; |
-
-
745076-30-6
C20H20N6OS3

-
A
-
10424-68-7
1,1'-(sulfonylbis(4,1-phenylene))bis(ethan-1-one)

-
B
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid at 20℃; for 168h; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 25℃; for 12h; Inert atmosphere; | 100% |
| In tetrahydrofuran at 5 - 20℃; for 1h; | 90% |
| In tetrahydrofuran at 20℃; | 88% |
-
-
85102-97-2
1-(2-Nitro-phenyl)-1H-imidazole-4-carbaldehyde

-
-
79-19-6
thiosemicarbazide

-
-
94128-89-9
C11H10N6O2S

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; Heating; | 100% |
-
-
85102-95-0
1-(3-Nitro-benzyl)-1H-imidazole-4-carbaldehyde

-
-
79-19-6
thiosemicarbazide

-
-
94128-92-4
C12H12N6O2S

| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; Heating; | 100% |
-
-
129994-30-5
2-(1,2,2,5,5-Pentamethyl-3-oxy-2,5-dihydro-1H-imidazol-4-yl)-1-phenyl-ethanone

-
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 1h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With acetic acid In water for 0.5h; Reflux; | 100% |
| In methanol | 90% |
| With hydrogenchloride In ethanol at 20℃; for 3h; | 90% |
-
-
79-19-6
thiosemicarbazide

-
-
72406-27-0
1-Formyl-3H-pyrrolo<2,3-c>carbazole

-
-
78706-10-2
1-Formyl-3H-pyrrolo<2,3-c>carbazole thiosemicarbazone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 3h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 24h; Condensation; | 100% |

| Conditions | Yield |
|---|---|
| In ethanol at 90℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane soln. of reagents in 1,4-dioxane was heated to 50°C under reflux; ppt. was filtered off, washed with 1,4-dioxane and air-dried, then dried over P2O5 under vac.; elem. anal.; | 100% |
-
-
79-19-6
thiosemicarbazide

-
-
93-02-7
2,5-dimethoxybenzaldehyde

-
-
329069-71-8
2,5-dimethoxybenzaldehyde thiosemicarbazone

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.25h; Milling; Green chemistry; | 100% |
| With acetic acid In ethanol for 24h; Reflux; | 82% |
| With sodium acetate In ethanol; water at 20℃; for 0.25h; | |
| In ethanol Reflux; |
-
-
1711-09-7
3-bromobenzoyl chloride

-
-
79-19-6
thiosemicarbazide

-
-
126651-84-1
2-(3-bromobenzoyl)hydrazinecarbothioamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 24h; Inert atmosphere; | 100% |
| With pyridine at 0℃; for 1h; |
-
-
124-04-9
Adipic acid

-
-
79-19-6
thiosemicarbazide

-
-
98558-04-4
5,5′-(butane-1,4-diyl)bis(1,3,4-thiadiazol-2-amine)

| Conditions | Yield |
|---|---|
| Stage #1: thiosemicarbazide With 1-ethyl-3-methylimidazolium hydrogensulfate at 50℃; for 0.25h; Stage #2: Adipic acid With sulfuric acid at 100℃; | 100% |
| With phosphorus pentachloride at 20℃; for 0.333333h; Time; Milling; | 95% |
| With trichlorophosphate for 5h; Reflux; | 76.9% |
| With trichlorophosphate at 110℃; for 5h; | 76.9% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; Milling; Green chemistry; | 100% |
| With sulfuric acid In ethanol Reflux; Cooling with ice; | 75% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 0.5h; Reflux; | 100% |
| With acetic acid In ethanol for 24h; Reflux; |

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 4h; Reflux; | 100% |
| With sulfuric acid In ethanol at 40℃; for 4h; | 96% |
-
-
950-81-2
4-formyl-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one

-
-
79-19-6
thiosemicarbazide

-
-
96715-43-4
4-formylantipyrine thiosemicarbazone

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; Milling; Green chemistry; | 100% |
| With acetic acid In ethanol for 3h; Reflux; |
-
-
1072-83-9
2-Acetylpyrrole

-
-
79-19-6
thiosemicarbazide

-
-
340737-11-3
2-[1-(pyrrol-2-yl)ethylidene]hidrazine carbothioamide

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 1h; Milling; Green chemistry; | 100% |
| With sulfuric acid In ethanol at 65℃; for 2.5h; | 51% |
| With acetic acid Reflux; | |
| With methanol |

-
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 60℃; for 3.5h; | 100% |
| With trifluoroacetic acid at 60℃; for 3.5h; |

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 0 - 78℃; for 3h; Inert atmosphere; | 100% |
-
-
92682-48-9
2-hydroxy-5-((2-methoxyphenyl)diazenyl)benzaldehyde

-
-
79-19-6
thiosemicarbazide

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol Reflux; | 99.91% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 40 - 50℃; for 1.5h; | 99.6% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 40 - 50℃; for 1.5h; | 99.6% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 40 - 50℃; for 1.5h; | 99.5% |
Thiosemicarbazide Chemical Properties
Structure of Thiosemicarbazide (CAS NO.79-19-6):
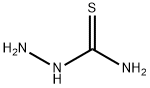
IUPAC Name: aminothiourea
Empirical Formula: CH5N3S
Molecular Weight: 91.1355
EINECS: 201-184-7
Index of Refraction: 1.666
Molar Refractivity: 24.63 cm3
Molar Volume: 66.2 cm3
Polarizability: 9.76× 10-24cm3
Surface Tension: 91 dyne/cm
Density: 1.376 g/cm3
Flash Point: 80 °C
Enthalpy of Vaporization: 44.49 kJ/mol
Melting Point: 180-183 °C (dec.)(lit.)
Boiling Point: 208.6 °C at 760 mmHg
Vapour Pressure: 0.212 mmHg at 25°C
Water Solubility: soluble
Stability: Stable. Incompatible with strong oxidizing agents.
Physical Appearance: Product Categories: Pharmaceutical Intermediates;organic sulfide
Thiosemicarbazide Toxicity Data With Reference
| 1. | dnd-hmn:hla 20 µmol/L | BCPCA6 Biochemical Pharmacology. 25 (1976),821. | ||
| 2. | orl-rat TDLo:1024 mg/kg/78W-C:ETA | JJIND8 JNCI, Journal of the National Cancer Institute. 67 (1981),75. | ||
| 3. | orl-rat LD50:9160 µg/kg | MarJV# Personal Communication from Josef V. Marhold, VUOS, 539-18, Pardubice, Czechoslovakia, to the Editor of RTECS, Cincinnati, OH, March 29, 1977 29MAR77 . | ||
| 4. | orl-mus LDLo:94 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 14 (1985),111. | ||
| 5. | ipr-mus LD50:1 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 6. | scu-mus LD50:16,407 µg/kg ABMGAJ 21,635,68 | |||
| 7. | ivn-mus LD50:13 mg/kg | JPETAB Journal of Pharmacology and Experimental Therapeutics. 122 (1958),110. | ||
| 8. | orl-dog LD50:10 mg/kg | HBTXAC Handbook of Toxicology, Volumes I-V. 5 (1959),155. | ||
| 9. | orl-cat LD50:20 mg/kg | PSEBAA Proceedings of the Society for Experimental Biology and Medicine. 70 (1949),688. | ||
| 10. | ipr-gpg LD50:24 mg/kg | PSEBAA & |
Thiosemicarbazide Consensus Reports
EPA Extremely Hazardous Substances List. Reported in EPA TSCA Inventory.
Thiosemicarbazide Safety Profile
Hazard Codes:T+ 
Risk Statements:28-52/53-26/27/28
R28:Very toxic if swallowed.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R26/27/28:Very toxic by inhalation, in contact with skin and if swallowed.
Safety Statements:22-26-36/37-45-61-28A-36/37/39
S22:Do not breathe dust.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S28:After contact with skin, wash immediately with plenty of soap-suds.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
RIDADR:UN 2811 6.1/PG 2
WGK Germany:3
RTECS:VT4200000
F:8-9-23
HazardClass:6.1
PackingGroup:II
HS Code:29309070
Poison by ingestion, intraperitoneal, and intravenous routes. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Thiosemicarbazide Specification
Thiosemicarbazide has the Synonyms of 1-Amino-2-thiourea; 1-Aminothiourea; 2-Thiosemicarbazide; 3-Thiosemicarbazide; Hydrazinecarbothioamide; N-Aminothiourea; NSC 2213; Semicarbazide, 3-thio-; Semicarbazide, thio-; Thiocarbamoylhydrazine; Thiocarbamylhydrazine. With the CAS NO.79-19-6, it is a white crystalline solid. Thiosemicarbazide is used for the synthesis of anti-TB drugs such as Thioacetazone and sulfa drugs. And it is used as a pesticide intermediate, rubber additives, synthetic resin additives and analytical reagent. It is used for the preparation of carbamate insecticides and thiadiazole fungicides and also used as a rodenticide intermediate.
Physical properties about Thiosemicarbazide are: (1)ACD/LogP: -1.155; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): -1.16; (4)ACD/LogD (pH 7.4): -1.16; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 5.61; (8)ACD/KOC (pH 7.4): 5.60; (9)#H bond acceptors: 3; (10)#H bond donors: 5; (11)#Freely Rotating Bonds: 1; (12)Index of Refraction: 1.667; (13)Molar Refractivity: 24.637 cm3; (14)Molar Volume: 66.201 cm3; (15)Polarizability: 9.767 10-24cm3; (16)Surface Tension: 91.0770034790039 dyne/cm; (17)Density: 1.377 g/cm3; (18)Flash Point: 79.953 °C; (19)Enthalpy of Vaporization: 44.485 kJ/mol; (20)Boiling Point: 208.59 °C at 760 mmHg; (21)Vapour Pressure: 0.211999997496605 mmHg at 25°C
When you are using this chemical, please be cautious about it as the following:
1. Do not breathe dust;
2. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
3. Wear suitable protective clothing and gloves;
4. In case of accident or if you feel unwell, seek medical advice immediately (show label where possible);
5. Avoid release to the environment. Refer to special instructions safety data sheet;
6. Wear suitable protective clothing, gloves and eye/face protection;
You can still convert the following datas into molecular structure:
(1)InChI=1S/CH5N3S/c2-1(5)4-3/h3H2,(H3,2,4,5);
(2)InChIKey=BRWIZMBXBAOCCF-UHFFFAOYSA-N;
(3)SmilesC(NN)(N)=S;
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 9100ug/kg (9.1mg/kg) | Toxicology and Applied Pharmacology. Vol. 21, Pg. 315, 1972. | |
| cat | LD50 | oral | 20mg/kg (20mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | Proceedings of the Society for Experimental Biology and Medicine. Vol. 70, Pg. 688, 1949. |
| dog | LD50 | oral | 10mg/kg (10mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 155, 1959. | |
| guinea pig | LD50 | intraperitoneal | 24mg/kg (24mg/kg) | GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Proceedings of the Society for Experimental Biology and Medicine. Vol. 70, Pg. 688, 1949. |
| mouse | LD50 | intraperitoneal | 1mg/kg (1mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | intravenous | 13mg/kg (13mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Pharmacology and Experimental Therapeutics. Vol. 122, Pg. 110, 1958. |
| mouse | LD50 | subcutaneous | 16407ug/kg (16.407mg/kg) | Acta Biologica et Medica Germanica. Vol. 21, Pg. 635, 1968. | |
| mouse | LDLo | oral | 94mg/kg (94mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 14, Pg. 111, 1985. | |
| rabbit | LD50 | oral | 13mg/kg (13mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 10, Pg. 686, 1960. | |
| rabbit | LD50 | skin | 2200mg/kg (2200mg/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. OTS0539438, |
| rabbit | LDLo | ocular | 50mg/kg (50mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA BLOOD: HEMORRHAGE | National Technical Information Service. Vol. OTS0539438, |
| rabbit | LDLo | subcutaneous | 14mg/kg (14mg/kg) | BEHAVIORAL: EXCITEMENT ENDOCRINE: HYPERGLYCEMIA | Journal of Pharmacology and Experimental Therapeutics. Vol. 30, Pg. 87, 1927. |
| rat | LD50 | oral | 9160ug/kg (9.16mg/kg) | Personal Communication from J.V. Marhold, VUOS, 539-18, Pardubice, Czechoslavakia, Mar. 29, 1977Vol. 29MAR1977, |
Related Products
- Thiosemicarbazide
- Thiosemicarbazide hydrochloride
- 79199-84-1
- 79200-56-9
- 79200-80-9
- 79201-39-1
- 79201-43-7
- 79206-94-3
- 79207-42-4
- 79208-53-0
- 79-20-9
- 79-21-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View