-
Name
Topotecan
- EINECS 231-595-7
- CAS No. 123948-87-8
- Article Data8
- CAS DataBase
- Density 1.49 g/cm3
- Solubility soluble in water
- Melting Point ?114 °C
- Formula C23H23N3O5
- Boiling Point 782.9 °C at 760 mmHg
- Molecular Weight 421.453
- Flash Point 427.3 °C
- Transport Information UN 3286 3/PG 2
- Appearance light yellow needle crystal or crystalline powder
- Safety 26-45-36/37/39-37/39
- Risk Codes 36/37/38-67-35-20-11-34
-
Molecular Structure
-
Hazard Symbols
 T,
T, C,
C, F,
F, Xi
Xi
- Synonyms 4-Ethyl-4,9-dihydroxy-10-[(dimethylamino)methyl]-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione;9-Dimethylaminomethyl-10-hydroxycamptothecin;(S)-10-((Dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione;1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione,10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-, (4S)-;
- PSA 104.89000
- LogP 1.84680
Synthetic route
-
-
50-00-0
formaldehyd

-
-
19685-09-7
(S)-10-hydroxycamptothecin

-
-
124-40-3
dimethyl amine

-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| With acetic acid Mannich reaction; | 65% |
| In acetic acid at 40 - 90℃; Mannich Aminomethylation; |

-
-
75-09-2
dichloromethane

-
-
19685-09-7
(S)-10-hydroxycamptothecin

-
-
124-40-3
dimethyl amine

-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| With potassium carbonate at 20℃; for 5h; Mannich reaction; | 65% |

-
-
7689-03-4
camptothecin

-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: H2; AcOH / PtO2 1.2: 51 percent / Pb(OAc)4; AcOH 2.1: 65 percent / aq. AcOH View Scheme |
-
-
19685-09-7
(S)-10-hydroxycamptothecin

-
-
30354-18-8
N,N-dimethyl(methylene)ammonium chloride

-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane; isopropyl alcohol at 20 - 35℃; for 12h; Mannich Reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: topotecan With 1H-imidazole In N,N-dimethyl-formamide for 0.166667h; Inert atmosphere; Stage #2: triethylsilyl chloride With dmap In N,N-dimethyl-formamide | 94% |
| With 1H-imidazole; dmap In N,N-dimethyl-formamide for 52.1667h; | 94% |
-
-
123948-87-8
topotecan

-
-
284049-22-5
tert-butyl (2-isocyanatoethyl)carbamate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide; acetonitrile at 0 - 20℃; for 2h; | 69% |
-
-
123948-87-8
topotecan

-
-
113238-61-2
tert-butyl 2-isocyanatoacetate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide; acetonitrile at 0 - 20℃; for 2h; | 68% |
-
-
123948-87-8
topotecan

-
-
145577-00-0
[11C]methyl triflate

| Conditions | Yield |
|---|---|
| In acetonitrile | 60% |
| In acetonitrile at -15 - 80℃; |
-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| With phosphate buffer Ambient temperature; other camptothecin lactone; also in the presence of DNA oligonucleotides; rate of hydrolysis; | |
| With poly(DL-lactide-co-glycolide); poly(vinyl alcohol) In various solvents at 37℃; for 192h; pH=7.4; Product distribution; Kinetics; Further Variations:; reaction times; Hydrolysis; | |
| With water at 37℃; |
-
-
123948-87-8
topotecan

-
-
119413-54-6, 123949-07-5
Topotecan hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 10 - 30℃; for 2h; Product distribution / selectivity; |
-
-
123948-87-8
topotecan

-
-
333-27-7
methyl trifluoromethanesulfonate

-
B
-
175613-38-4
C21H18N2O6

-
C
-
1243272-93-6
CHF3O3S*C23H23N3O5

| Conditions | Yield |
|---|---|
| Stage #1: methyl trifluoromethanesulfonate With potassium carbonate In dichloromethane for 0.5h; Stage #2: topotecan In dichloromethane at 20℃; for 24h; |

-
-
66-27-3
Methyl methanesulfonate

-
-
123948-87-8
topotecan

-
A
-
175613-38-4
C21H18N2O6

-
D
-
123948-98-1
10-hydroxy-9-<(trimethylammonio)methyl>-(20S)-camptothecin methanosulfonate

| Conditions | Yield |
|---|---|
| Stage #1: Methyl methanesulfonate With potassium carbonate In dichloromethane for 0.5h; Stage #2: topotecan In dichloromethane at 20℃; for 24h; |

-
-
123948-87-8
topotecan

-
-
386768-13-4, 189397-70-4, 112269-92-8
25,26,27,28-terahydroxycalix[4]arene-5,11,7,23-tetrasulfonic acid

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; for 48h; |
-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| Stage #1: topotecan With isopropylamine Stage #2: With pyridine; acetic anhydride Stage #3: With ammonia In dichloromethane | |
| Stage #1: topotecan With isopropylamine Stage #2: With pyridine; acetic anhydride Stage #3: With ammonia In dichloromethane |
-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dmap; dicyclohexyl-carbodiimide / dichloromethane / 66 h / 20 °C 2: trifluoroacetic acid / dichloromethane / 1 h / 20 °C View Scheme |
-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 24 h / Heating 2: lithium hydroxide; water / tetrahydrofuran / 6 h / 20 °C View Scheme |
-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 24 h / Heating 2: lithium hydroxide; water / tetrahydrofuran / 6 h / 20 °C 3: 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine / N,N-dimethyl-formamide / 0 - 20 °C / Inert atmosphere View Scheme |
-
-
108-30-5
succinic acid anhydride

-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| With pyridine at 50℃; for 48h; | 0.35 g |
-
-
123948-87-8
topotecan

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / 48 h / 50 °C 2: 1,1'-carbonyldiimidazole; 1,8-diazabicyclo[5.4.0]undec-7-ene / dimethyl sulfoxide / 24 h / 20 °C View Scheme |
Topotecan Specification
1. Introduction of Topotecan
The Topotecane with CAS registry number of 123948-87-8, has the systematic name is (4S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione. It belongs to product categories of APIs. This chemical is a light yellow needle crystal or crystalline powder and it can be used as anti-cancer drugs.
2. Properties of Topotecan
Physical properties about Topotecane are: (1)ACD/LogP: 1.08; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.06; (4)ACD/LogD (pH 7.4): 0.45; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 21.36; (9)#H bond acceptors: 8; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 5; (12)Polar Surface Area: 81.2Å2; (13)Index of Refraction: 1.733; (14)Molar Refractivity: 112.73 cm3; (15)Molar Volume: 281.3 cm3; (16)Polarizability: 44.69×10-24cm3; (17)Surface Tension: 82.8 dyne/cm; (18)Density: 1.49 g/cm3; (19)Flash Point: 427.3 °C; (20)Enthalpy of Vaporization: 119.49 kJ/mol; (21)Boiling Point: 782.9 °C at 760 mmHg; (22)Vapour Pressure: 8.13E-26 mmHg at 25 °C
3. Structure Descriptors of Topotecan
You could convert the following datas into the molecular structure:
1). Canonical SMILES: CCC1(C2=C(COC1=O)C(=O)N3CC4=C(C3=C2)N=C5C=CC(=C(C5=C4)CN(C)C)O)O
2). Isomeric SMILES: CC[C@@]1(C2=C(COC1=O)C(=O)N3CC4=C(C3=C2)N=C5C=CC(=C(C5=C4)CN(C)C)O)O
3). InChI: InChI=1S/C23H23N3O5/c1-4-23(30)16-8-18-20-12(9-26(18)21(28)15(16)11-31-22(23)29)7-13-14(10-25(2)3)19(27)6-5-17(13)24-20/h5-8,27,30H,4,9-11H2,1-3H3/t23-/m0/s1
4). InChIKey: UCFGDBYHRUNTLO-QHCPKHFHSA-N
4. Preparation of Topotecan
Preparation of Topotecane: it is prepared by reaction of 37% formaldehyde with 40% dimethylamine. Besides, The reaction needs reagent acetic acid.
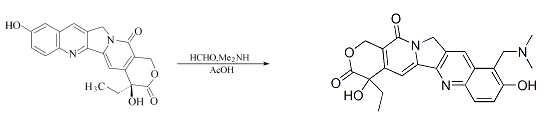
5. Safety Information of Topotecan
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. Besides, it has highly flammable and may causes severe burns. What's more, it is harmful by inhalation. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you feel unwell or accident happens seek medical advice immediately.
Related Products
- Topotecan
- Topotecan acetate
- Topotecan hydrochloride
- 123950-44-7
- 123950-46-9
- 1239513-63-3
- 123-95-5
- 123-96-6
- 123970-57-0
- 12397-06-7
- 123973-22-8
- 123973-25-1
- 123982-81-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View