-
Name
Triethyl orthopropionate
- EINECS 204-108-0
- CAS No. 115-80-0
- Article Data12
- CAS DataBase
- Density 0.898 g/cm3
- Solubility soluble in alcohol ether, slightly soluble in water
- Melting Point 284 °C
- Formula C9H20O3
- Boiling Point 171 °C at 760 mmHg
- Molecular Weight 176.256
- Flash Point 60 °C
- Transport Information UN 3272 3/PG 3
- Appearance clear colorless liquid
- Safety 26
- Risk Codes 36/38-22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Triethyl o-propionate;1,1,1-triethoxypropane;Propane, 1,1, 1-triethoxy-;Orthopropionic acid, triethyl ester;Orthopropionic acid, triethyl ester (8CI);Triethylorthopropionate;Triethyl Ortho Propionate;Orthopropionic acid ethyl ester;Ethyl orthopropionate;propane, 1,1,1-triethoxy-;
- PSA 27.69000
- LogP 2.15970
Synthetic route
-
-
64-17-5
ethanol

-
-
21504-43-8
1,1-di-ethoxyprop-1-ene

-
-
105-56-6
ethyl 2-cyanoacetate

-
B
-
115-80-0
Triethyl orthopropionate

| Conditions | Yield |
|---|---|
| Stage #1: 1,1-di-ethoxyprop-1-ene; ethyl 2-cyanoacetate at 80℃; for 1h; Stage #2: ethanol | A n/a B 40% |


| Conditions | Yield |
|---|---|
| (i) NaOEt, (ii) AcOH; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| at 25℃; | |
| With diethyl ether |
-
-
7647-01-0
hydrogenchloride

-
-
64-17-5
ethanol

-
-
21504-43-8
1,1-di-ethoxyprop-1-ene

-
-
115-80-0
Triethyl orthopropionate

-
-
91895-02-2
(6-fluoro-3-methoxyquinoxalin-2-yl)hydrazine

-
-
115-80-0
Triethyl orthopropionate
-
-
91895-10-2
6-chloro-2-hydrazino-3-methoxyquinoxaline

-
-
115-80-0
Triethyl orthopropionate
-
-
96134-79-1
3-<(Dimethylamino)sulfonyl>benzoic Acid Hydrazide

-
-
115-80-0
Triethyl orthopropionate

-
-
96134-51-9
3-(5-Ethyl-[1,3,4]oxadiazol-2-yl)-N,N-dimethyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| 100% |
-
-
107-19-7
propargyl alcohol

-
-
115-80-0
Triethyl orthopropionate

-
-
60523-21-9
ethyl 2-methyl-3,4-pentadienoate

| Conditions | Yield |
|---|---|
| With acetic acid Johnson-Claisen rearrangement; Heating; | 100% |
| With propionic acid at 100 - 160℃; for 2h; | 85% |
| With propionic acid In neat (no solvent) at 100 - 153℃; | 77% |
| With propionic acid at 150 - 160℃; | 70% |
| propionic acid at 140 - 145℃; |
-
-
335394-55-3
(22R,23E)-26-methyl-6β-methoxy-3α,5-cyclo-5α-27-norcholest-23-en-22-ol

-
-
115-80-0
Triethyl orthopropionate

| Conditions | Yield |
|---|---|
| With propionic acid In toluene at 140℃; for 0.75h; Johnson orthoester Claisen rearrangement; | 100% |
-
-
361193-21-7
2-(3-nitrophenyl)acetic hydrazide

-
-
115-80-0
Triethyl orthopropionate

-
-
1308256-63-4
2-ethyl-5-(3-nitrobenzyl)-1,3,4-oxadiazole

| Conditions | Yield |
|---|---|
| With acetic acid for 3h; Reflux; | 100% |

-
-
115-80-0
Triethyl orthopropionate

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In 1,2-dichloro-ethane Heating; optical yield given as %ee; | 100% |

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In 1,2-dichloro-ethane at 65℃; for 1h; Inert atmosphere; | 100% |
| With pyridinium p-toluenesulfonate In 1,2-dichloro-ethane at 65℃; for 2h; |
-
-
434285-80-0
N4-(4-((tert-butyldimethylsilyl)oxy)butyl)quinoline-3,4-diamine

-
-
115-80-0
Triethyl orthopropionate

| Conditions | Yield |
|---|---|
| In toluene for 20h; Reflux; | 100% |
| In toluene for 20h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol; toluene | A n/a B 99.5% |

-
-
2222-19-7
ethyl 8-hydroxy-2-oxo-2H-cycloheptafuran-3-carboxylate

-
-
115-80-0
Triethyl orthopropionate

-
-
134919-97-4
2,4-Diethoxy-3-methyl-azulene-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Heating; | 99% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
115-80-0
Triethyl orthopropionate

-
-
205178-47-8
(4R)-2-ethyl-4-phenyl-2-oxazoline

| Conditions | Yield |
|---|---|
| With acetic acid In 1,2-dichloro-ethane for 2h; Cyclization; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With propionic acid In toluene Johnson-Claisen rearrangement; Reflux; | 99% |
-
-
26961-27-3
2-amino-4,5-dimethoxybenzonitrile

-
-
115-80-0
Triethyl orthopropionate

-
-
1088610-92-7
ethyl N-(4,5-dimethoxy-2-cyanophenyl)propanimidate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid | 99% |
-
-
115-80-0
Triethyl orthopropionate

-
-
1885-29-6
anthranilic acid nitrile

-
-
132121-43-8
ethyl N-(2-cyanophenyl)propanimidate

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid | 99% |

-
-
115-80-0
Triethyl orthopropionate

-
-
1449104-65-7
(E)-ethyl 5-cyclopentyl-2,4-dimethylpent-4-enoate

| Conditions | Yield |
|---|---|
| With 2-Ethylhexanoic acid; 2,6-di-tert-butyl-4-methyl-phenol In toluene at 195 - 200℃; for 24h; Autoclave; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In n-heptane at 100℃; under 67506.8 Torr; Temperature; | 98.6% |
-
-
613-94-5
benzoic acid hydrazide

-
-
115-80-0
Triethyl orthopropionate

-
-
73314-40-6
2-ethyl-5-phenyl-1,3,4-oxadiazole

| Conditions | Yield |
|---|---|
| With ammonium chloride In ethanol for 0.75h; Reflux; | 98% |
| With sulfuric acid; silica gel at 20℃; | 93% |
| With potassium aluminum sulfate at 100℃; for 6h; | 92% |
| With Nafion(R)NR50 at 80℃; for 0.166667h; microwave irradiation; | 88% |
-
-
16867-03-1
2-amino-3-hydroxypyridine

-
-
115-80-0
Triethyl orthopropionate

-
-
52333-88-7
2-ethyloxazolo<4,5-b>pyridine

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 85℃; for 0.166667h; | 98% |
| With bismuth(lll) trifluoromethanesulfonate at 85℃; for 0.1h; | 85% |
| With toluene-4-sulfonic acid at 140 - 180℃; | 30% |
| With toluene-4-sulfonic acid at 20 - 180℃; | 30% |

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 20℃; for 0.0333333h; | 98% |
| With tris(trifluoroacetato)bismuth(III) at 20℃; for 0.0666667h; | 97% |
| With 1-(propyl-3-sulfonate) 3-methylimidazol(3H)-1-ium phosphotungstate In water at 20℃; for 0.25h; Reagent/catalyst; Time; | 96% |
-
-
53617-66-6
3-cyano-2H-cycloheptafuran-2-one

-
-
115-80-0
Triethyl orthopropionate

-
-
134919-77-0
2-Ethoxy-3-methyl-azulene-1-carbonitrile

| Conditions | Yield |
|---|---|
| Heating; | 98% |

| Conditions | Yield |
|---|---|
| With propionic acid | 98% |
-
-
199169-93-2
(22S,23Z)-6-(1,3-dioxolan-2-yl)-3α,5-cyclo-26,27-bisnor-5α-cholest-23-en-22-ol

-
-
115-80-0
Triethyl orthopropionate

-
-
199169-94-3
(22E,24R)-6-(1,3-dioxolan-2-yl)-24-methyl-3α,5-cyclo-5α-cholest-22-en-26-oic acid ethyl ester

| Conditions | Yield |
|---|---|
| With propionic acid In xylene for 2h; Heating; | 98% |
-
-
492-41-1
(1R,2S)-norephedrine

-
-
115-80-0
Triethyl orthopropionate

-
-
205178-48-9
(4S,5R)-2-ethyl-4-methyl-5-phenyl-2-oxazoline

| Conditions | Yield |
|---|---|
| With acetic acid In 1,2-dichloro-ethane for 2h; Cyclization; Heating; | 98% |
-
-
3182-95-4
L-Phenylalaninol

-
-
115-80-0
Triethyl orthopropionate

-
-
75866-73-8
(S)-2-ethyl-4,5-dihydro-4-(phenylmethyl)oxazole

| Conditions | Yield |
|---|---|
| With acetic acid In 1,2-dichloro-ethane for 2h; Cyclization; Heating; | 98% |
| With acetic acid In 1,2-dichloro-ethane at 115 - 125℃; for 2h; | 93% |
-
-
27259-73-0
2-(2'-aminophenyl)-4(3H)-quinazolinone

-
-
115-80-0
Triethyl orthopropionate

-
-
109588-56-9
6-ethylquinazolino[4,3-b]quinazolin-8-one

| Conditions | Yield |
|---|---|
| for 0.05h; microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With tris(trifluoroacetato)bismuth(III) at 20℃; for 0.00833333h; | 98% |
| With 1-(propyl-3-sulfonate) 3-methylimidazol(3H)-1-ium phosphotungstate In water at 20℃; for 0.133333h; Reagent/catalyst; Time; | 97% |
| With tin dioxide In ethanol at 20℃; for 0.2h; Green chemistry; | 96% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 20℃; for 0.0333333h; | 90% |
-
-
95-84-1
3-amino-4-hydroxytoluene

-
-
115-80-0
Triethyl orthopropionate

-
-
20514-29-8
2-ethyl-5-methylbenzo[d]oxazole

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at 20℃; for 0.1h; | 98% |
| With 1-(propyl-3-sulfonate) 3-methylimidazol(3H)-1-ium phosphotungstate In water at 20℃; for 0.166667h; Reagent/catalyst; Time; | 97% |
| With tris(trifluoroacetato)bismuth(III) at 20℃; for 0.0416667h; | 95% |
| With tin dioxide In ethanol at 20℃; for 0.3h; Green chemistry; | 95% |
| With silica supported fluoroboric acid at 20℃; for 0.833333h; Neat (no solvent); | 94% |
-
-
104873-63-4
(22R)-6β-methoxy-3α,5-cyclo-5α-chol-23-yn-22-ol

-
-
115-80-0
Triethyl orthopropionate

| Conditions | Yield |
|---|---|
| With propionic acid In benzene for 5h; Johnson-Claisen Rearrangement; Reflux; Inert atmosphere; diastereoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With tungstate sulfuric acid In neat (no solvent) at 80 - 90℃; for 0.0666667h; Reagent/catalyst; | 98% |
-
-
109-77-3
malononitrile

-
-
115-80-0
Triethyl orthopropionate

-
-
35260-96-9
2-(1-ethoxypropylidene)malononitrile

| Conditions | Yield |
|---|---|
| In acetic anhydride for 15h; Reflux; | 97% |
| With acetic anhydride for 15h; Reflux; | 97% |
-
-
22442-46-2
ethyl 2-oxo-2H-cyclohepta[b]furan-3-carboxylate

-
-
115-80-0
Triethyl orthopropionate

-
-
129612-94-8
2-Ethoxy-3-methyl-azulene-1-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Heating; | 97% |
Triethyl orthopropionate Specification
The Triethyl orthopropionate, with the CAS registry number 115-80-0 and EINECS registry number 204-108-0, has the systematic name of 1,1,1-triethoxypropane. And the molecular formula of this chemical is C9H20O3. It is a kind of clear colorless liquid, and belongs to the following product categories: Intermediates of Dyes and Pigments; straight chain compounds; Orthoesters; Acetals/Ketals/Ortho Esters; Organic Building Blocks; Oxygen Compounds. What's more, it is often used as analytical reagent and film sensitizer, and it is also used in organic synthesis, dyeing industry and pharmaceuticals industry.
The physical properties of Triethyl orthopropionate are as following: (1)ACD/LogP: 3.03; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.03; (4)ACD/LogD (pH 7.4): 3.03; (5)ACD/BCF (pH 5.5): 117.5; (6)ACD/BCF (pH 7.4): 117.5; (7)ACD/KOC (pH 5.5): 1055.2; (8)ACD/KOC (pH 7.4): 1055.2; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 7; (12)Polar Surface Area: 27.69 Å2; (13)Index of Refraction: 1.413; (14)Molar Refractivity: 48.94 cm3; (15)Molar Volume: 196.2 cm3; (16)Polarizability: 19.4×10-24cm3; (17)Surface Tension: 26.2 dyne/cm; (18)Density: 0.898 g/cm3; (19)Flash Point: 60 °C; (20)Enthalpy of Vaporization: 39.07 kJ/mol; (21)Boiling Point: 171 °C at 760 mmHg; (22)Vapour Pressure: 1.9 mmHg at 25°C.
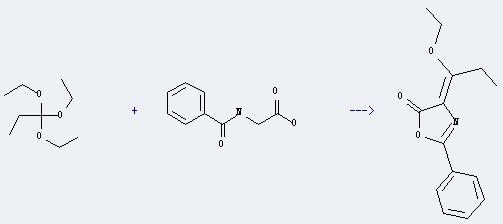
Uses of Triethyl orthopropionate: It can react with N-benzoyl-glycine to produce 4-(1-ethoxy-propylidene)-2-phenyl-4H-oxazol-5-one. This reaction will need reagents 4-(dimethylamino)pyridine and acetic anhydride. The reaction time is 1 hour with heating, and the yield is about 48%.
You should be cautious while dealing with this chemical. It irritates eyes and skin, and it is also harmful if swallowed. Therefore, in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O(CC)C(OCC)(OCC)CC
(2)InChI: InChI=1/C9H20O3/c1-5-9(10-6-2,11-7-3)12-8-4/h5-8H2,1-4H3
(3)InChIKey: FGWYWKIOMUZSQF-UHFFFAOYAI
Related Products
- Triethyl 1,1,2-ethanetricarboxylate
- Triethyl 1,3,5-benzenetricarboxylate
- Triethyl 2-chloro-2-phosphonoacetate
- Triethyl 2-fluoro-2-phosphonoacetate
- Triethyl 2-phosphonopropionate
- Triethyl 3-bromopropane-1,1,1-tricarboxylate
- Triethyl acetyl citrate
- Triethyl Orthoformate
- Triethyl orthopropionate
- Triethyl Phosphate
- 1158098-73-7
- 115810-12-3
- 115812-96-9
- 115816-31-4
- 1158-17-4
- 115822-58-7
- 115822-61-2
- 115823-68-2
- 115826-95-4
- 115-83-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View