-
Name
Trimethyl phosphate
- EINECS 208-144-8
- CAS No. 512-56-1
- Article Data152
- CAS DataBase
- Density 1.154 g/cm3
- Solubility 500 G/L (25 ºC)
- Melting Point -46 °C
- Formula C3H9O4P
- Boiling Point 197.2 °C at 760 mmHg
- Molecular Weight 140.076
- Flash Point 83.7 °C
- Transport Information UN 2810
- Appearance clear liquid
- Safety 53-36/37-45-36/37/39
- Risk Codes 46-40-68-45-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, T
T
- Synonyms Methylphosphate, (MeO)3PO (6CI);Trimethoxyphosphineoxide;Trimethyl orthophosphate;Trimethylphosphoric acid;
- PSA 54.57000
- LogP 1.03370
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hypophosphite; copper dichloride at 24.9℃; for 173h; | 100% |
| With phosphorous; phosphoric acid tributyl ester; oxygen; copper dichloride In benzene at 30℃; | 98.2% |
| With trichlorophosphate In acetone at 10 - 30℃; under 10 Torr; for 4h; | 97.5% |

| Conditions | Yield |
|---|---|
| With sulfur trioxide In dichloromethane at -95℃; | 100% |
| With dihydrogen peroxide In pyridine at -20℃; adding different FeIII compounds; determination of half-lives of reactions; | |
| With naphthalene-1,4-dicarbonitrile; water In water; acetonitrile Irradiation; |

| Conditions | Yield |
|---|---|
| With sulfur trioxide In dichloromethane at -50℃; | A 7% B 93% |
-
-
122203-30-9
dimethyl (Z)-α-methoxyiminobenzylphosphonate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| at 140℃; for 0.5h; Product distribution; reflux in 1,2,4-trimethylbenzene, 72 h; | A 85% B 92% |
-
-
67-56-1
methanol

-
-
7357-14-4
p-methoxyphenyl dimethyl phosphate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
150-76-5
4-methoxy-phenol

| Conditions | Yield |
|---|---|
| With cesium fluoride at 65℃; for 22h; | A 79% B 88% |

-
-
67-56-1
methanol

-
-
74532-26-6, 74532-27-7
1-decenyl dimethyl phosphate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
112-31-2
caprinaldehyde

| Conditions | Yield |
|---|---|
| With cesium fluoride at 65℃; for 22h; | A 67% B 88% |

-
-
7357-14-4
p-methoxyphenyl dimethyl phosphate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
150-76-5
4-methoxy-phenol

| Conditions | Yield |
|---|---|
| With methanol; cesium fluoride at 65℃; for 22h; | A 79% B 88% |
-
-
74532-26-6, 74532-27-7
1-decenyl dimethyl phosphate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
112-31-2
caprinaldehyde

| Conditions | Yield |
|---|---|
| With methanol; cesium fluoride at 65℃; for 22h; | A 67% B 88% |
-
-
67-56-1
methanol

-
A
-
512-56-1
trimethyl phosphite

-
B
-
812-00-0
methylphosphate

-
C
-
813-78-5
dimethylphosphoric acid

| Conditions | Yield |
|---|---|
| With phosphorus; tetraethylammonium iodide In water; acetonitrile electrolysis; | A 82% B n/a C n/a |

-
-
67-56-1
methanol

-
-
10113-28-7
dimethyl phenyl phosphate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With cesium fluoride at 65℃; for 4h; | A 80% B 65% |


| Conditions | Yield |
|---|---|
| With methanol; cesium fluoride at 65℃; for 4h; | A 80% B 65% |

| Conditions | Yield |
|---|---|
| With cesium fluoride at 65℃; for 0.5h; | A 63% B 76% |
| With La-(catecholate)-functionalized porous organic polymer at 60℃; for 12h; Kinetics; Reagent/catalyst; | |
| Stage #1: methanol With UiO-66-H for 0.5h; Stage #2: methyl paraoxon at 59.84℃; |


| Conditions | Yield |
|---|---|
| With methanol; cesium fluoride at 65℃; for 0.5h; | A 63% B 76% |
-
-
31142-23-1
trimethyl phosphonoformate

-
A
-
512-56-1
trimethyl phosphite

-
B
-
756-79-6
dimethyl methane phosphonate

| Conditions | Yield |
|---|---|
| at 295℃; for 6h; | A 18% B 75% |
-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
512-56-1
trimethyl phosphite

-
B
-
96-36-6, 868-85-9
methyl phosphite

| Conditions | Yield |
|---|---|
| With Oxone In tetrahydrofuran; methanol Ambient temperature; | A 72% B n/a |
-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
512-56-1
trimethyl phosphite

-
B
-
152-18-1
O,O,O-trimethylthiophosphate

-
C
-
756-79-6
dimethyl methane phosphonate

| Conditions | Yield |
|---|---|
| With sulfur trioxide In dichloromethane at -95℃; | A 25% B 4% C 71% |


| Conditions | Yield |
|---|---|
| With phosphorous; 10H-phenothiazine; N,N,N,N-tetraethylammonium tetrafluoroborate In N,N-dimethyl-formamide at 53℃; electrolysis; | A 9% B 71% |
-
-
67-56-1
methanol

-
A
-
512-56-1
trimethyl phosphite

-
B
-
868-85-9
Dimethyl phosphite

-
C
-
756-79-6
dimethyl methane phosphonate

| Conditions | Yield |
|---|---|
| With phosphorous; tetraethylammonium iodide In acetonitrile at 18℃; electrolysis; | A 70% B 11% C 3% |
| With phosphorous; tetraethylammonium iodide In acetonitrile at 18℃; Mechanism; Product distribution; electrolysis; var. nucleophiles; var. temperatures; | A 70% B 11% C 3% |
| With phosphorous In acetonitrile at 18℃; Electrolysis; | A 70% B 11% C 3% |


| Conditions | Yield |
|---|---|
| With Oxone In tetrahydrofuran; methanol Ambient temperature; | 70% |
| With 3-chloro-benzenecarboperoxoic acid at -25℃; Yield given; | |
| With ozone at 50℃; for 2.5h; neat (no solvent); |

| Conditions | Yield |
|---|---|
| With tetraethylammonium iodide; water In acetonitrile at 48℃; electrosynthesis; | A 65% B 5% |
-
-
591-87-7
Allyl acetate

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
512-56-1
trimethyl phosphite

-
B
-
757-54-0
allylphosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate In 1,4-dioxane at 145 - 160℃; for 15h; | A 17% B 64% |


-
A
-
512-56-1
trimethyl phosphite

-
-
25125-72-8
trans-2,2-Diphenylaziridine

| Conditions | Yield |
|---|---|
| With methanol In dichloromethane at 40℃; for 0.5h; | A n/a B 62% |
-
-
67-56-1
methanol

-
-
74783-15-6
C10H19O8P

-
A
-
512-56-1
trimethyl phosphite

-
B
-
4148-97-4
dimethyl 2-methoxybutanedioate

-
C
-
115-10-6
Dimethyl ether

| Conditions | Yield |
|---|---|
| at 150℃; for 20h; Product distribution; reaction under var. conditions; | A 60% B n/a C n/a D 40% |
| at 150℃; for 2h; |


-
A
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran | A n/a B 10% C 57% |

-
-
699-18-3, 32782-45-9
2-(2-nitrovinyl)furan

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
512-56-1
trimethyl phosphite

-
B
-
2697-42-9
phosphoramidic acid dimethyl ester

| Conditions | Yield |
|---|---|
| In acetic acid at 40℃; for 24h; | A 16 g B 5 g C 55% |
| In acetic acid at 40℃; for 24h; Product distribution; Mechanism; | A 16 g B 5 g C 10 g |
| In acetic acid at 40℃; for 24h; | A 16 g B 5 g C 10 g |

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
512-56-1
trimethyl phosphite

-
B
-
152-18-1
O,O,O-trimethylthiophosphate

-
C
-
77-78-1
dimethyl sulfate

-
D
-
756-79-6
dimethyl methane phosphonate

| Conditions | Yield |
|---|---|
| With sulfur trioxide In dichloromethane at -78℃; Product distribution; other trialkyl phosphite,trialkyl phosphine, trialkyl arsines, trialkoxyarsines, var. molar ratio and temperatures; | A 22% B 4% C 55% D 19% |
| With sulfur trioxide In dichloromethane at -78℃; | A 48% B 16% C 3% D 9% |


| Conditions | Yield |
|---|---|
| With phosphorous; tetraethylammonium iodide In acetonitrile at 50℃; electrolysis; | A 51% B 28% |
| With phosphorous In acetonitrile at 50℃; Electrolysis; | A 51% B 28% |
-
-
67-56-1
methanol

-
-
920-66-1
1,1,1,3',3',3'-hexafluoro-propanol

-
A
-
512-56-1
trimethyl phosphite

-
B
-
19784-21-5
O,O-dimethyl O-(1,1,1,3,3,3-hexafluoropropan-2-yl) phosphate

| Conditions | Yield |
|---|---|
| Stage #1: 1,1,1,3',3',3'-hexafluoro-propanol With phosphorus pentachloride for 2h; Heating; Stage #2: methanol | A 2% B 6% C 12% D 50% |

-
-
121-45-9
phosphorous acid trimethyl ester

-
A
-
512-56-1
trimethyl phosphite

-
B
-
7211-39-4
dimethyl phosphine oxide

| Conditions | Yield |
|---|---|
| With Bromotrichloromethane In neat (no solvent) at 25℃; for 0.5h; | A 4% B 50% |
-
-
152-18-1
O,O,O-trimethylthiophosphate

-
-
19602-00-7
3-methylpyridazine-2-oxide

-
A
-
1632-76-4
3-methylpyridazine

-
B
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| In dichloromethane for 5h; Product distribution; Irradiation; different thiophosphoryl compounds, its concentrations and reaction times; | A 43% B 27% |

-
-
512-56-1
trimethyl phosphite

-
-
119750-30-0
3-Hydroxy-7,8-dimethyl-1-oxo-2-phenyl-1,5-dihydro-benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile

-
-
119750-43-5
3-Methoxy-5,7,8-trimethyl-1-oxo-2-phenyl-1,5-dihydro-benzo[4,5]imidazo[1,2-a]pyridine-4-carbonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate for 1.5h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| at 20 - 140℃; for 4h; | 100% |
| In acetonitrile at 80℃; for 24h; Inert atmosphere; | 99% |
| at 100℃; for 24h; | 95% |
-
-
512-56-1
trimethyl phosphite

-
-
79917-90-1
1-butyl-3-methylimidazolium chloride

| Conditions | Yield |
|---|---|
| at 100℃; for 2h; | 100% |
| at 60℃; for 5h; | 100% |

| Conditions | Yield |
|---|---|
| at 100℃; for 3h; | 100% |
-
-
7098-07-9
N-Ethylimidazole

-
-
512-56-1
trimethyl phosphite

-
-
945611-27-8
1-ethyl-3-methyl-1H-imidazol-3-ium dimethyl phosphate

| Conditions | Yield |
|---|---|
| In neat liquid at 80℃; for 18h; Reflux; Inert atmosphere; | 100% |
| at 80℃; for 24h; Inert atmosphere; | 99% |
| In tetrahydrofuran Reflux; | |
| at 150℃; for 15h; Inert atmosphere; | |
| at 149.99℃; for 10h; |
-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| at 50℃; for 0.25h; Microwave irradiation; | 100% |
| With calcium chloride In toluene at 110℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With cyclopentyl methyl ether; sodium hydroxide at 115℃; for 24h; Reagent/catalyst; Inert atmosphere; | 100% |
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; Reagent/catalyst; Solvent; Temperature; regioselective reaction; | 99% |
-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 120℃; for 4h; | 99% |
-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 100℃; for 8h; | 99% |
-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 120℃; for 2h; | 99% |
-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 110℃; for 4h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
945611-42-7
1-butyl-2,3-dimethylimidazolium dimethyl phosphate

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 100℃; for 2h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
67-48-1
choline chloride

-
-
118978-98-6
(2-hydroxyethyl)trimethylammonium dimethyl phosphate

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 120℃; for 2h; | 99% |
| In methanol at 80℃; for 12h; |
-
-
512-56-1
trimethyl phosphite

-
-
1323125-78-5
1-butyl-1-methylpyrrolidinium dimethyl phosphate

| Conditions | Yield |
|---|---|
| With calcium chloride In toluene at 110℃; for 2h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
459409-74-6
4,4,5,5-tetramethyl-2-(5-phenylthiophen-2-yl)-1,3,2-dioxaborolane

-
-
5069-26-1
2-methyl-5-phenylthiophene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; lithium iodide; lithium tert-butoxide at 50℃; for 16h; Inert atmosphere; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
3389-53-5
phenylacetylpyrrolidine

-
-
74931-56-9
2-phenyl-1-(pyrrolidin-1-yl)propan-1-one

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
17123-83-0
N-(phenylacetyl)morpholine

-
-
155222-99-4
1-(4-morpholinyl)-2-phenyl-1-propanone

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
18925-69-4
N,N-dimethyl-2-phenylacetamide

-
-
81616-82-2, 81616-83-3, 41836-85-5
N,N-dimethyl-2-phenylpropionamide

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
588-46-5
N-(phenylmethyl)acetamide

-
-
29823-47-0
N-benzyl-N-methylacetamide

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
151647-54-0
3-phenyl-1-pyrrolidin-1-yl-propane-1-one

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 75℃; for 24h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
5830-31-9
N,N-dimethyl-3-phenylpropanamide

-
-
20929-40-2
2-methyl-N,N-dimethyl-3-phenylpropanamide

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at 0 - 75℃; for 24h; | 99% |
-
-
512-56-1
trimethyl phosphite

-
-
153392-54-2
tetrahydro-2'-methyl-3'-phenylspiro(cyclopentane-1,5'-isoxazole)

| Conditions | Yield |
|---|---|
| at 160℃; for 0.5h; | 98% |
-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| at 160℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 24h; Inert atmosphere; | 98% |
| In ethyl acetate at 80℃; Inert atmosphere; | 76.5% |
| In tetrahydrofuran Reflux; | |
| at 110℃; for 24h; Inert atmosphere; |
-
-
512-56-1
trimethyl phosphite

-
-
109-64-8
1,3-dibromo-propane

-
-
177342-84-6
(3-bromopropyl)phosphonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| at 150℃; for 0.5h; | 98% |
-
-
512-56-1
trimethyl phosphite

-
-
21252-69-7
N-octylimidazole

-
-
945611-33-6
1-octyl-3-methylimidazolium dimethyl phosphate

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 24h; Inert atmosphere; | 98% |

-
-
512-56-1
trimethyl phosphite

| Conditions | Yield |
|---|---|
| With calcium chloride In acetonitrile for 2h; Reflux; | 98% |
Trimethyl phosphate Consensus Reports
.
Trimethyl phosphate Specification
The IUPAC name of this chemical is Trimethyl phosphate. With the CAS registry number 512-56-1 and EINECS registry number 208-144-8, it is also named as Phosphoric acid,trimethyl ester. In addition, the molecular formula is C3H9O4P and the molecular weight is 140.07. It is a kind of clear liquid and belongs to the classes of Organics; Functional Materials; Phosphates (Plasticizer); Plasticizer; Organic Building Blocks; Organic Phosphates/Phosphites; Phosphorus Compounds.
Physical properties about this chemical are: (1)ACD/LogP: -0.52; (2)ACD/LogD (pH 5.5): -0.52; (3)ACD/LogD (pH 7.4): -0.52; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 12.46; (7)ACD/KOC (pH 7.4): 12.46; (8)#H bond acceptors: 4; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 54.57 Å2; (11)Index of Refraction: 1.379; (12)Molar Refractivity: 28.05 cm3; (13)Molar Volume: 121.2 cm3; (14)Polarizability: 11.12 ×10-24cm3; (15)Surface Tension: 29.4 dyne/cm; (16)Density: 1.154 g/cm3; (17)Flash Point: 83.7 °C; (18)Enthalpy of Vaporization: 41.57 kJ/mol; (19)Boiling Point: 197.2 °C at 760 mmHg; (20)Vapour Pressure: 0.539 mmHg at 25°C.
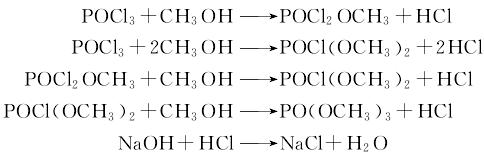
Preparation of Trimethyl phosphate: it can be prepared by methanol and trichlorooxyphosphorus in the presence of potassium carbonate. Add methanol and potassium carbonate into reactor at reaction temperature of 5 °C. Then add trichlorooxyphosphorus into the mixture in 2 hours with stirring. The temperature should be controlled below 30 °C. Then add dimethyl sulfate and reflux for 3 hours. At last, you can go through the operation of cooling, filtering by carbon tetrachloride, vacuum distillation and drying to get the products.
Uses of Trimethyl phosphate: this chemical is a mild methylating agent, useful for preparing dimethylation of anilines and related heterocyclic compounds.And it is mianly used as solvent and extractant for medicine and pesticide. Also it can be used as a color inhibitor for fibers, flame retardants and plasticizer. In addition, it can react with pyridine to get 1-methyl-pyridinium; dimethyl phosphate. The reaction time is 5 hours by heating. The yield is about 92%.
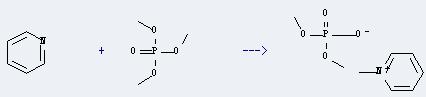
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. And it may cause heritable genetic damage and cancer. In addition, it has risk of irreversible effects Possibly. There is limited evidence of a carcinogenic effect. Avoid exposure - obtain special instruction before use. During using it, wear suitable protective clothing, gloves and eye/face protection. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: O=P(OC)(OC)OC
(2)InChI: InChI=1/C3H9O4P/c1-5-8(4,6-2)7-3/h1-3H3
(3)InChIKey: WVLBCYQITXONBZ-UHFFFAOYAV
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 1676mg/kg (1676mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 1129, 1986. | |
| mouse | LD50 | intraperitoneal | 2250mg/kg (2250mg/kg) | Therapie. Vol. 15, Pg. 237, 1960. | |
| mouse | LD50 | oral | 1470mg/kg (1470mg/kg) | Progress Report for Contract No. NIH-NCI-E-C-72-3252, Submitted to the National Cancer Institute by Litton Bionetics, Inc. Vol. NCI-E-C-72-3252, Pg. 1973, | |
| mouse | LD50 | unreported | 540mg/kg (540mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Gigiena Naselennykh Mest. Hygiene in Populated Places. Vol. 17, Pg. 37, 1978. |
| quail | LD50 | oral | 750mg/kg (750mg/kg) | Journal of Reproduction and Fertility. Vol. 48, Pg. 371, 1976. | |
| rabbit | LD50 | oral | 1275mg/kg (1275mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Pharmacology and Experimental Therapeutics. Vol. 88, Pg. 338, 1946. |
| rabbit | LD50 | skin | 2830uL/kg (2.83mL/kg) | American Industrial Hygiene Association Journal. Vol. 30, Pg. 470, 1969. | |
| rat | LD50 | oral | 840mg/kg (840mg/kg) | Progress Report for Contract No. NIH-NCI-E-C-72-3252, Submitted to the National Cancer Institute by Litton Bionetics, Inc. Vol. NCI-E-C-72-3252, Pg. 1973, | |
| rat | LD50 | unreported | 500mg/kg (500mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Gigiena Naselennykh Mest. Hygiene in Populated Places. Vol. 17, Pg. 37, 1978. |
| rat | LDLo | intraperitoneal | 800mg/kg (800mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC GASTROINTESTINAL: OTHER CHANGES | Journal of Pharmacy and Pharmacology. Vol. 11, Pg. 150, 1959. |
| rat | LDLo | intravenous | 2400mg/kg (2400mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Nature. Vol. 179, Pg. 154, 1957. |
Related Products
- Trimethyl indium
- Trimethyl lead chloride
- Trimethyl N-(p-benzenesulphonamido)phosphorimidate
- Trimethyl nonanone
- Trimethyl orthopropionate
- Trimethyl orthovalerate
- Trimethyl phosphate
- Trimethyl phosphite
- Trimethyl phosphonoacetate
- Trimethyl phosphorotrithioate
- 51260-39-0
- 51-26-3
- 512-63-0
- 51263-08-2
- 512-64-1
- 51264-14-3
- 51268-87-2
- 512-69-6
- 51269-82-0
- 51273-56-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View