-
Name
TRIMETHYLINDIUM
- EINECS 222-200-9
- CAS No. 3385-78-2
- Article Data37
- CAS DataBase
- Density 1.568 g/cmg/cm3
- Solubility
- Melting Point 88 °C
- Formula C3H9In
- Boiling Point 135.7°C
- Molecular Weight 159.924
- Flash Point -18°C
- Transport Information
- Appearance COA
- Safety 7-16-43-45
- Risk Codes 14-17-34
-
Molecular Structure
- Hazard Symbols
- Synonyms Trimethylindium;
- PSA 0.00000
- LogP 1.75140
Synthetic route

| Conditions | Yield |
|---|---|
| With methylene chloride; sodium at 30 - 40℃; under 375.038 Torr; Flow reactor; Inert atmosphere; | 93% |
-
-
10025-82-8
indium(III) chloride

-
-
7439-95-4
magnesium

-
-
74-88-4
methyl iodide

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In diethyl ether a Grignard soln. (prepd. from MeI and Mg in Et2O) is added to the suspn. of InCl3 in ether and refluxed for 3 h; distn. of excess of Et2O at atm. pressure affords Me3In*Et2O; repeated distn. of the etherate at room temp. in vac. affords InMe3; | 92% |


-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In neat (no solvent) metal complex decomposed at 110-135°C for 2-3 h; | 81% |

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In neat (no solvent) metal complex decomposed at 110-135°C for 2-3 h; | 75% |

| Conditions | Yield |
|---|---|
| at 70℃; | 66% |

| Conditions | Yield |
|---|---|
| With indium(III) chloride | |
| With indium(III) chloride In tetrahydrofuran at -78℃; Cooling with dry ice bath; |

| Conditions | Yield |
|---|---|
| With mercury dichloride at 100℃; |
-
-
331815-63-5
tetrakis(trimethylindium) 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane adduct

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80-115°C under vac.; |
-
-
329955-33-1
trimethylindium bis(N,N,N',N'-tetramethyl-4,4'-methylenebis(aniline)) adduct

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80-115°C under vac.; |
-
-
331815-64-6
hexakis(trimethylindium) 1,4,7,10,13,16-hexamethyl-1,4,7,10,13,16-hexaazacyclooctadecane adduct

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80-115°C under vac.; |

| Conditions | Yield |
|---|---|
| With mercury dichloride heating for 8 days at 100°C in CO2 atmosphere; cooled by ice then Hg(CH3)2 removed by distillation; | |
| With HgCl2 heating for 8 days at 100°C in CO2 atmosphere; cooled by ice then Hg(CH3)2 removed by distillation; |

| Conditions | Yield |
|---|---|
| In diethyl ether InCl3 heated at 80°C in vac. for 1 h, purged Ar, ether added, cooled (ice bath), MeLi added; stirred for 2 h, evapd.; | |
| In diethyl ether (inert atmosphere); addn. of MeLi in Et2O to InCl3 in Et2O at 0°C, warming to room temp.; filtration; prepd. as soln.; | |
| In not given under Ar; MeLi (3 equiv.) in THF or hexane or Et2O added slowly (15-30 min) to cooled (-78°C) soln. of InCl3 in THF; stirred for 30 min; warmed to room temp.; according to H. C. Clark, A. L. Pickard, J. Organomet. Chem. 8 (1967) 427; |

-
A
-
7440-69-9
bismuth

-
B
-
7440-74-6
indium

-
C
-
81174-93-8
tetrakis(trimethylsilyl)dibismutane

-
D
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In not given decompn. at temp. above -30 °C within minutes; |


-
A
-
67605-92-9
dimethylbis(η5-pentamethylcyclopentadienyl)uranium

-
B
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In neat (no solvent) drying under vac.; |

| Conditions | Yield |
|---|---|
| Stage #1: indium; methyl iodide In 2,6,10,15,19,23-hexamethyltetracosane at 110℃; for 2h; Stage #2: trimethylaluminum With tri-n-propylamine In 2,6,10,15,19,23-hexamethyltetracosane at 90℃; for 1h; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78℃; for 0.5h; | |
| In tetrahydrofuran at -78℃; for 0.5h; | |
| In tetrahydrofuran; diethyl ether at 0 - 20℃; for 0.25h; Inert atmosphere; |

-
A
-
3385-78-2
trimethyl indium

-
B
-
14629-99-3
dimethylindium chloride

| Conditions | Yield |
|---|---|
| With potassium chloride; sodium chloride at 150 - 250℃; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| With Durasyn 162 for 3.5h; Temperature; | 92 %Spectr. |

| Conditions | Yield |
|---|---|
| In 2-Methylpentane at 20 - 38℃; | 100 %Spectr. |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane at -78℃; |

| Conditions | Yield |
|---|---|
| In toluene under Ar or N2, stirred, refluxed for 2-3 h; removal of solvent, filtered off, washed with benzene or hexane, dried under vac.; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In toluene molar ratio 3:1; | 100% |
| In benzene dry Ar atm.; 3 equiv. of AlMe3, cooling (-15°C), heating (room temp., stirring), refluxing (1,5 h, 70-80°C); concn., standing (5 to -5 degree.C, several days), washing (toluene), drying (vac.); elem. anal.; | 70% |

| Conditions | Yield |
|---|---|
| In toluene molar ratio 3:1; | 100% |

| Conditions | Yield |
|---|---|
| In toluene molar ratio 3:1; | 100% |
| In benzene dry Ar atm.; 3 equiv. of AlMe3, cooling (-15°C), heating (room temp., stirring), refluxing (1,5 h, 70-80°C); concn., standing (5 to -5 degree.C, several days), washing (toluene), drying (vac.); elem. anal.; | 84% |

| Conditions | Yield |
|---|---|
| In toluene molar ratio 3:1; | 100% |
| In benzene dry Ar atm.; 3 equiv. of AlMe3, cooling (-15°C), heating (room temp., stirring), refluxing (1,5 h, 70-80°C); concn., standing (5 to -5 degree.C, several days), washing (toluene), drying (vac.); elem. anal.; | 79% |
-
-
234443-33-5
[(CH3)2SnN(CH2CH(CH3)2)]3

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In toluene molar ratio 3:1; | 100% |
| In benzene dry Ar atm.; 3 equiv. of AlMe3, cooling (-15°C), heating (room temp., stirring), refluxing (1,5 h, 70-80°C); concn., standing (5 to -5 degree.C, several days), washing (toluene), drying (vac.); elem. anal.; | 88% |

| Conditions | Yield |
|---|---|
| In toluene molar ratio 3:1; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 12h; Inert atmosphere; Schlenk technique; | 100% |
| In benzene Inert atmosphere; Schlenk technique; |
-
-
18166-43-3
tris(tert-butoxy)silanol

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 12h; Inert atmosphere; Schlenk technique; | 100% |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran | 99% |

| Conditions | Yield |
|---|---|
| In hexane; toluene under Ar, 1 equiv. of metal-compd. was added to 9:1 hexane:toluene soln.of amine; crystn. at -30 °C; elem. anal.; | 99% |
| In diethyl ether; Petroleum ether soln. of equimolar amts. of aminopyridine and Me3In in diethyl ether/light petroleum (1:2) cooled to -30°C; crystals collected, washed with light petroleum, and dried in vac.; elem. anal.; | 91% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In toluene (N2); dropwise addn. of InMe3 to methyl salicylate in toluene at -78°C, warming to room temp.; solvent removal (vac.), crystn. (toluene/hexane); elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| In pentane (N2); filtn., stirring; solvent removal (vac.); | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane byproducts: CH4; elem. anal.; | 99% |
-
-
136030-00-7
(1R,2S)-1-Amino-2-indanol

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; all manipulations under dry oxygen-free N2 atm.; soln. of org. compd. added dropwise to soln. of metal compd. at room temp., stirred for 12 h; solvent removed in vac., crystd. from toluene; elem. anal.; | 99% |
-
-
3385-78-2
trimethyl indium

-
-
7480-35-5, 13286-59-4, 74165-73-4, 126456-43-7, 136030-00-7, 140632-19-5, 140632-20-8
(1S,2R)-1-amino-2-indanol

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; all manipulations under dry oxygen-free N2 atm.; soln. of org. compd. added dropwise to soln. of metal compd. at room temp., stirred for 12 h; solvent removed in vac., crystd. from toluene; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CH4; (Ar); addn. of ligand to indium compd. at -196°C, slow warming toroom temp., keeping at 20°C for 28 d; evapn.; | 98.3% |
-
-
27720-03-2
(S)-2-dimethylamino-3-phenyl-1-propanol

-
-
3385-78-2
trimethyl indium

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; under N2, 1 equiv. of alc. in toluene was added to a stirred toluene soln. of Me3In at room temp., stirring for 0.5 h; solvent was removed in vac., crystn. from n-pentane at 6 °C, elem. anal.; | 98% |
Trimethyl indium Specification
The Trimethyl indium with CAS registry number of 3385-78-2 is also called Indium,trimethyl-. Its EINECS registry number is 222-200-9. The IUPAC name is trimethylindigane. In addition, the molecular formula is C3H9In and the molecular weight is 159.92.
Physical properties about this chemical are: (1)Exact Mass: 159.974303; (2)MonoIsotopic Mass: 159.974303; (3)Heavy Atom Count: 4; (4)Complexity: 8; (5)Covalently-Bonded Unit Count: 1;.
Preparation of Trimethyl indium: it can be prepared by In, Mg and CH3I. You can go through the operation of solution collaterals and fractionation to get high purity Trimethyl indium.
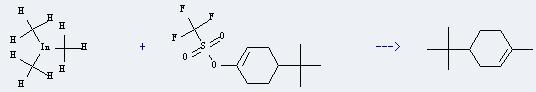
Uses of Trimethyl indium: it can react with 1-trifluoromethansulfonyloxy-4-t-butylcyclohexene to get 1-methyl-4-tert-butyl-1-cyclohexene. This reaction will need reagent Pd(PPh3)2Cl2 and solvent tetrahydrofuran. The reaction time is 7 hours by heating. The yield is about 92%.
When you are using this chemical, please be cautious about it as the following:
It is spontaneously flammable in air and can react violently with water. And it can cause burns. In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water). And in case of accident or if you feel unwell, seek medical advice immediately (show label where possible). You should keep container tightly closed and keep away from sources of ignition - No smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: [In](C)(C)C
(2)InChI: InChI=1/3CH3.In/h3*1H3;/rC3H9In/c1-4(2)3/h1-3H3
(3)InChIKey: IBEFSUTVZWZJEL-SGQDGSKVAB
Related Products
- Trimethyl arsine
- TRIMETHYL BENZENE
- Trimethyl benzene-1,3,5-tricarboxylate
- TRIMETHYL BISMUTH
- Trimethyl indium
- Trimethyl lead chloride
- Trimethyl N-(p-benzenesulphonamido)phosphorimidate
- Trimethyl nonanone
- Trimethyl orthopropionate
- Trimethyl orthovalerate
- 33858-36-5
- 3386-00-3
- 338-61-4
- 3386-18-3
- 3386-33-2
- 33863-76-2
- 3386-42-3
- 338-64-7
- 33864-99-2
- 338-69-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View