-
Name
Trioctylphosphine oxide
- EINECS 201-121-3
- CAS No. 78-50-2
- Article Data33
- CAS DataBase
- Density 0.861 g/cm3
- Solubility insoluble in water
- Melting Point 50-55°C
- Formula C24H51OP
- Boiling Point 408.842 °C at 760 mmHg
- Molecular Weight 386.642
- Flash Point 259.779 °C
- Transport Information
- Appearance white crystalline powder
- Safety 26-36/39-39
- Risk Codes 38-41
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Cyanex 921;Hostarex PX 324;TOPO;Tri-n-octylphosphine oxide;Trioctylphosphine oxide;
- PSA 26.88000
- LogP 9.43100
Synthetic route

| Conditions | Yield |
|---|---|
| With fluorosulfonylchloride In dichloromethane for 1h; Ambient temperature; | 98% |
| Stage #1: TOP With water; dihydrogen peroxide In toluene at 0 - 20℃; for 12h; Inert atmosphere; Stage #2: In toluene at 20℃; for 4h; Molecular sieve; | 90% |
| In 1,2,4-trimethylbenzene at 25℃; for 1h; Inert atmosphere; | |
| With selenium; octadec-1-ene |

| Conditions | Yield |
|---|---|
| In chloroform at 50℃; for 2h; | 95.1% |

-
-
78-50-2
cyanex-921

| Conditions | Yield |
|---|---|
| With water In toluene at 20℃; for 1h; | 92.6% |
-
-
4731-53-7
TOP

-
-
22585-81-5
4-chlorophenylphosphonoyl dichloride

-
A
-
78-50-2
cyanex-921

-
B
-
1005-33-0
4-chlorophenyldichlorophosphine

| Conditions | Yield |
|---|---|
| In dichloromethane | A n/a B 84% |


| Conditions | Yield |
|---|---|
| With acetic acid at 120 - 135℃; for 8h; | 81.4% |

| Conditions | Yield |
|---|---|
| With sodium amide; tert-butyl alcohol In tetrahydrofuran at 40℃; for 0.833333h; | 72% |
-
-
106446-20-2
3-(4-chlorophenyl)-4-trioctylphosphiniminofurazan

-
A
-
78-50-2
cyanex-921

-
B
-
106446-23-5
azoxy(4-chlorophenylfurazan)

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In 1,2-dichloro-ethane for 2h; Heating; | A n/a B 60% |

| Conditions | Yield |
|---|---|
| In toluene; Petroleum ether | 39% |
| With hydrogenchloride; trichlorophosphate In 5,5-dimethyl-1,3-cyclohexadiene; diethyl ether; water |
-
-
111-83-1
1-bromo-octane

-
A
-
3011-82-3
oxyde de dioctyl phosphine

-
B
-
78-50-2
cyanex-921

-
C
-
6196-68-5
octylphosphinic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide semihydrate; phosphorus; N-benzyl-N,N,N-triethylammonium chloride In toluene at 60 - 62℃; for 5h; Reagent/catalyst; Inert atmosphere; | A n/a B n/a C 19% |

-
-
17049-49-9
octylmagnesium bromide

-
-
78-50-2
cyanex-921

| Conditions | Yield |
|---|---|
| With diethyl ether; trichlorophosphate | |
| With trichlorophosphate |
-
-
111-85-3
1-Chlorooctane

-
-
1779-48-2
phenylphosphinic acid

-
A
-
78-50-2
cyanex-921

-
B
-
2845-09-2
dioctyl(phenyl)phosphine oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 200 deg C, 24 h, 2.) reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
111-85-3
1-Chlorooctane

-
-
107694-27-9
octylphenylphosphinous acid

-
A
-
78-50-2
cyanex-921

-
B
-
2845-09-2
dioctyl(phenyl)phosphine oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 200 deg C, 24 h, 2.) reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With phosphorus; iodine 1) 125 - 130 deg C, 36 h, 2) H2O; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
629-27-6
1-Iodooctane

-
A
-
683-19-2
dioctylphosphinic acid

-
B
-
78-50-2
cyanex-921

-
D
-
128144-48-9
1-methylheptyl-di-n-octylphosphine oxide

| Conditions | Yield |
|---|---|
| With phosphorus; iodine 1) 150 - 160 deg C, 16 h, 2) H2O; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide 1.) 200 deg C, 7.5 h, 2.) reflux, 4 h; Yield given. Multistep reaction; |
-
-
111-83-1
1-bromo-octane

-
-
107694-27-9
octylphenylphosphinous acid

-
A
-
78-50-2
cyanex-921

-
B
-
2845-09-2
dioctyl(phenyl)phosphine oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) 200 deg C, 5 h, 2.) reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With phosphorus; potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In tetrahydrofuran at 60 - 65℃; for 6h; Yield given; |
-
-
106446-20-2
3-(4-chlorophenyl)-4-trioctylphosphiniminofurazan

-
A
-
78-50-2
cyanex-921

-
B
-
106446-22-4
3-(4-chlorophenyl)-4-nitrofurazan

-
C
-
106446-23-5
azoxy(4-chlorophenylfurazan)

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In acetone for 16h; Ambient temperature; |


-
-
78-50-2
cyanex-921

| Conditions | Yield |
|---|---|
| With trichlorophosphate Alkylation; |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; Inert atmosphere; Schlenk technique; |


| Conditions | Yield |
|---|---|
| In chloroform at 20℃; Inert atmosphere; Schlenk technique; |

-
-
67206-59-1
sodium dioctylphosphinate

-
-
78-50-2
cyanex-921

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: chloroform / 16 h / 80 °C 2: chloroform / 2 h / 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: chloroform / 16 h / 80 °C 2: water / chloroform 3: acetic acid / 8 h / 120 - 135 °C View Scheme | |
| Multi-step reaction with 3 steps 1: chloroform / 16 h / 80 °C 2: toluene / 24 h / 100 °C 3: water / toluene / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hypophosphite; acetic acid / 4 h / 120 - 135 °C 2: chloroform / 16 h / 80 °C 3: chloroform / 2 h / 50 °C View Scheme | |
| Multi-step reaction with 4 steps 1: sodium hypophosphite; acetic acid / 4 h / 120 - 135 °C 2: chloroform / 16 h / 80 °C 3: water / chloroform 4: acetic acid / 8 h / 120 - 135 °C View Scheme | |
| Multi-step reaction with 4 steps 1: sodium hypophosphite; acetic acid / 4 h / 120 - 135 °C 2: chloroform / 16 h / 80 °C 3: toluene / 24 h / 100 °C 4: water / toluene / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: toluene / 24 h / 100 °C 2: water / toluene / 1 h / 20 °C View Scheme |
-
-
127440-67-9, 127641-61-6
MoO2Br2(H2O)2

-
-
78-50-2
cyanex-921

| Conditions | Yield |
|---|---|
| In diethyl ether mixed; evapd.(vac. room temp.), elem. anal.; | 99% |
-
-
78-50-2
cyanex-921

-
-
21741-98-0
3-amino-4-(p-chlorophenyl)-1,2,5-oxadiazole

-
-
106446-20-2
3-(4-chlorophenyl)-4-trioctylphosphiniminofurazan

| Conditions | Yield |
|---|---|
| With trifluoromethanesulfonic acid anhydride In dichloromethane 1.) -5 deg C, 0.5 h; 2.) -5 deg C, 3 h, then room temperature, overnight; | 93% |

| Conditions | Yield |
|---|---|
| In water suspending MoO3 in aq. H2O2 at 40°C for 4 h, addn. of the ligand,kept for 2 h; concn. (vac.), extn. (CH2Cl2), evapn., washing (H2O), drying (vac.); elem. anal.; | 93% |


| Conditions | Yield |
|---|---|
| In water; benzene UO2(NO3)2 soln. at pH 2 extd. with benzene soln. of PMAP and TOPO; benzene layer dried with Na2SO4, evapd. dryness in rotary evaporator and product recrystd. twice from n-hexane; elem. anal.; | 89% |


| Conditions | Yield |
|---|---|
| In chloroform at 20℃; Inert atmosphere; Schlenk technique; | 81.7% |
-
-
78-50-2
cyanex-921

-
-
1366181-66-9
[(κ2-P,O-2-(bis(2-methoxyphenyl)phosphino)benzenesulfonato)PdMe(OPOct3)]

| Conditions | Yield |
|---|---|
| With AgBF4 In dichloromethane byproducts: AgCl, NaBF4; using Schlenk techniques; stirring of suspn. of ((κ2-P,O-2-(2-MeOC6H4)2PC6H4SO3)PdCl(μ-Na))2 (0.5 equiv.), AgBF4 (1 equiv.) and OPOct3 (1 equiv.) in CH2Cl2 for 30 min in the dark; pptn., filtration, evapn., washing with pentane, drying under vac.; elem. anal.; | 81% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; Inert atmosphere; Schlenk technique; | 80.8% |

-
-
78-50-2
cyanex-921

-
-
90076-65-6
bis(trifluoromethane)sulfonimide lithium

| Conditions | Yield |
|---|---|
| Stage #1: cyanex-921; bis(trifluoromethane)sulfonimide lithium In ethanol at 60℃; Stage #2: europium(III) nitrate hexahydrate In ethanol; water at 60℃; for 4h; | 76% |


| Conditions | Yield |
|---|---|
| In ethanol at 20 - 50℃; for 21h; | 75% |


| Conditions | Yield |
|---|---|
| In 1,4-dioxane W compd. addn. to P-compd. soln. with stirring; oily phase sepn., washing (water, MeOH), solvent elimination (heating, 50°C, vac.), solidification (refrigerator); elem. anal.; | 73% |

| Conditions | Yield |
|---|---|
| In ethanol at 20 - 50℃; for 21h; | 71% |


| Conditions | Yield |
|---|---|
| In ethanol at 20 - 50℃; for 21h; | 68% |


| Conditions | Yield |
|---|---|
| In ethanol; water addition of 0.05 g Eu salt (as nitrate hydrate) in H2O (10 ml) to 0.08 g of the dione and 0.078 g of the phosphine oxide in 10 ml ethanol and solvation of the precipitate by addition of ethanol with stirring;; crystallization on standing in a beaker overnight; elem. anal., detn. by (1)H NMR-, IR spectroscopy, X-ray diffraction;; | 60% |


| Conditions | Yield |
|---|---|
| In chloroform-d1 Solvent; | 60% |

| Conditions | Yield |
|---|---|
| With pinacolboronic acid In acetonitrile at 100℃; for 120h; Inert atmosphere; Glovebox; Sealed tube; Schlenk technique; | 57% |
| With 1,1,3,3-Tetramethyldisiloxane; titanium(IV) isopropylate In various solvent(s) at 100℃; for 10h; | |
| With indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane In toluene at 100℃; for 18h; Inert atmosphere; Sealed tube; |
-
-
148509-02-8
(meso-5,10,15,20-tetra-p-tolylporphyrinato)Ti(η2-3-hexyne)

-
-
78-50-2
cyanex-921

-
-
326474-31-1
(meso-5,10,15,20-tetra-p-tolylporphyrinato)bis(trioctylphosphine oxide)Ti

| Conditions | Yield |
|---|---|
| In toluene byproducts: 3-hexyne; under N2; Ti complex and 2 equiv. of ligand in toluene stirred for 2.5 h; concd.; redissolved in hexanes; reduced in vac.; cooled to -25°C for 1 d; elem. anal.; | 52% |

| Conditions | Yield |
|---|---|
| In pentane (N2); addn. of 1 equiv. of boron compd. to a soln. of phosphine in pentane; elem. anal.; | 51% |

| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: ClCH(CH3)2; addn. of phosphine oxide to soln. of Ti-halide in heptadecane, heating (N2-atmosphere, 300°C), rapid addn. of 1 equiv. of Ti-alkoxide, 5 min; centrifugation, washing (acetone); XRD pattern characterisation; | 50% |


| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: BrCH(CH3)2; addn. of phosphine oxide to soln. of Ti-halide in heptadecane, heating (N2-atmosphere, 300°C), rapid addn. of 1 equiv. of Ti-alkoxide, 5 min; centrifugation, washing (acetone); XRD pattern characterisation; | 50% |


| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: FCH(CH3)2; addn. of phosphine oxide to soln. of Ti-halide in heptadecane, heating (N2-atmosphere, 300°C), rapid addn. of 1 equiv. of Ti-alkoxide, 5 min; centrifugation, washing (acetone); XRD pattern characterisation; | 50% |


| Conditions | Yield |
|---|---|
| In further solvent(s) byproducts: ICH(CH3)2; addn. of phosphine oxide to soln. of Ti-halide in heptadecane, heating (N2-atmosphere, 300°C), rapid addn. of 1 equiv. of Ti-alkoxide, 5 min; centrifugation, washing (acetone); XRD pattern characterisation; | 50% |

Trioctylphosphine oxide Specification
The IUPAC name of Tri-n-octylphosphine oxide is 1-Dioctylphosphoryloctane. With the CAS registry number 78-50-2, it is also named as Trioctyl phosphine oxide. The product's category is Phosphine Oxides and Sulfides, and the other registry number is 129406-23-1. Besides, it is white crystalline powder, which should be stored in closed, cool and dry place. In addition, this chemical is stable and incompatible with strong oxidizing agents, but insoluble in water.
The other characteristics of this product can be summarized as: (1)EINECS: 201-121-3; (2)ACD/LogP: 10.25; (3)# of Rule of 5 Violations: 1; (4)ACD/LogD (pH 5.5): 10.251; (5)ACD/LogD (pH 7.4): 10.251; (6)ACD/BCF (pH 5.5): 1000000; (7)ACD/BCF (pH 7.4): 1000000; (8)ACD/KOC (pH 5.5): 8984523; (9)ACD/KOC (pH 7.4): 8984523; (10)H bond acceptors: 1; (11)H bond donors: 0; (12)Freely Rotating Bonds: 21; (13)Index of Refraction: 1.448; (14)Molar Refractivity: 120.111 cm3; (15)Molar Volume: 448.845 cm3; (16)Surface Tension: 30.999 dyne/cm; (17)Density: 0.861 g/cm3; (18)Flash Point: 259.779 °C; (19)Melting point: 50-55 °C; (20)Enthalpy of Vaporization: 63.523 kJ/mol; (21)Boiling Point: 408.842 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
Preparation of Tri-n-octylphosphine oxide: this chemical can be prepared by Trioctylphosphane.
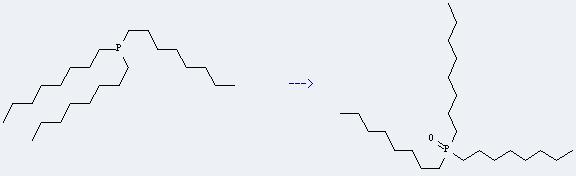
This reaction needs sulfuryl chloride fluoride and CH2Cl2 at ambient temperature. The reaction time is 1 hour. The yield is 98 %.
Uses of Tri-n-octylphosphine oxide: this chemical is used as extracting agent and solvent. And it also can react with 4-(4-Chloro-phenyl)-furazan-3-ylamine to get 3-(4-Chlorophenyl)-4-trioctylphosphiniminofurazan.

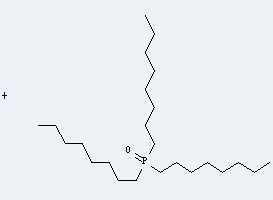
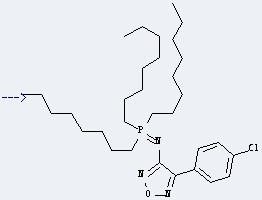
This reaction needs Trifluoromethanesulfonic anhydride and CH2Cl2 at temperature of -5 °C for 0.5 h. The yield is 93 %.
When you are using this chemical, please be cautious about it as the following: it is irritating to skin. And it is also risk of serious damage to the eyes. You should wear suitable protective clothing. and eye / face protection. Moreover, in case of contact with eyes, please rinse immediately with plenty of water and seek medical advice.
People can use the following data to convert to the molecule structure.
(1)SMILES:CCCCCCCCP(=O)(CCCCCCCC)CCCCCCCC
(2)InChI:InChI=1/C24H51OP/c1-4-7-10-13-16-19-22-26(25,23-20-17-14-11-8-5-2)24-21-18-15-12-9-6-3/h4-24H2,1-3H3
(3)InChIKey:ZMBHCYHQLYEYDV-UHFFFAOYAY
(4)Std. InChI:InChI=1S/C24H51OP/c1-4-7-10-13-16-19-22-26(25,23-20-17-14-11-8-5-2)24-21-18-15-12-9-6-3/h4-24H2,1-3H3
(5)Std. InChIKey:ZMBHCYHQLYEYDV-UHFFFAOYSA-N
The toxicity data is as follows:
| 1. | skn-rbt 500 µL/24H MOD | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) OTS0534743 . | ||
| 2. | eye-rbt 100 mg/24H SEV | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) OTS0572000 . |
Related Products
- Trioctylphosphine
- Trioctylphosphine oxide
- 78502-79-1
- 78502-81-5
- 78503-38-5
- 78508-96-0
- 78510-19-7
- 78512-39-7
- 78-51-3
- 78515-16-9
- 78515-84-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View