-
Name
Triphenylmethanol
- EINECS 200-988-5
- CAS No. 76-84-6
- Article Data607
- CAS DataBase
- Density 1.13 g/cm3
- Solubility insoluble in water
- Melting Point 160-163 °C(lit.)
- Formula C19H16O
- Boiling Point 380 °C at 760 mmHg
- Molecular Weight 260.335
- Flash Point 168.7 °C
- Transport Information
- Appearance white powder
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Methanol,triphenyl- (8CI);BL 3756;Hydroxytriphenylmethane;NSC 4050;Triphenylcarbinol;Triphenylmethyl alcohol;Tritanol;Tritylalcohol;U 45483;a,a-Diphenylbenzenemethanol;
- PSA 20.23000
- LogP 3.97080
Synthetic route

| Conditions | Yield |
|---|---|
| With water In tetrachloromethane | 100% |
| With water Kinetics; Rate constant; Mechanism; Two-phase systems; | |
| With water In acetone at 25℃; Kinetics; ΔS, ΔH (excit.); |
-
-
596-43-0
bromo-triphenyl-methane

-
-
691-88-3
di-sec-butyl mercury

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
53213-46-0, 868-82-6
secondary-butyl mercury(II) bromide

-
C
-
78504-79-7
sec-butyl triphenylmethyl peroxide

| Conditions | Yield |
|---|---|
| With oxygen; tetrabutylammomium bromide In 1,2-dichloro-ethane; benzene Product distribution; other reaction conditions; | A n/a B n/a C 100% |
| With oxygen; tetrabutylammomium bromide In 1,2-dichloro-ethane Yields of byproduct given; | A n/a B n/a C 76% |

-
-
50653-07-1
trimethylsilyl triphenyl methyl ether

-
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsilyl triphenyl methyl ether With nitrogen dioxide at 20℃; for 0.833333h; Stage #2: With water | 100% |
| With methanol; 1,3-disulfonic acid imidazolium hydrogen sulfate at 20℃; for 0.0833333h; Green chemistry; | 99% |
| With montmorillonite K-10 In methanol for 4h; Ambient temperature; | 98% |

-
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| With acid-washed bentonite In acetone at 40 - 50℃; for 0.166667h; | 100% |

| Conditions | Yield |
|---|---|
| With chromyl chloride In tetrachloromethane at 22℃; for 0.5h; ultrasound sonication; | 99% |
| With potassium hydroxide; 18-crown-6 ether; oxygen; dimethyl sulfoxide In 1,2-dimethoxyethane at 20℃; for 1.5h; | 97% |
| With chromium(VI) oxide; tetrabutylammonium periodite In dichloromethane; acetonitrile at -40℃; for 0.166667h; | 97% |
-
-
18909-18-7
1-diphenylmethylene-4-trityl-2,5-cyclohexadiene

-
A
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; acetonitrile at 20℃; for 0.5h; Reagent/catalyst; Inert atmosphere; Glovebox; | A 99% B 83% |

-
-
77134-74-8
4,5-dihydroxymethyl-1-triphenylmethylimidazole

-
A
-
33457-48-6
4,5-dihydroxymethylimidazole

-
B
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| With acetic acid for 1.5h; Heating; | A 98.5% B n/a |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 50℃; for 12h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| In water at 25℃; for 0.00555556h; Solvent; | 98% |

| Conditions | Yield |
|---|---|
| With potassium 12-tungstocobaltate(III) In water; acetonitrile for 0.2h; Microwave irradiation; | 96% |
| With 1H-imidazole; C17H16ClMnN2O2; tetrabutylammonium periodite In chloroform at 20℃; for 0.333333h; | 95% |
| With oxygen; mercury(II) oxide In methanol; acetonitrile at 25℃; UV-irradiation; | 88% |
-
-
83075-47-2
methyl 2,4-di-O-benzyl-3-O-methyl-6-O-trityl-α-D-galactopyranoside

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
83075-48-3
methyl 2,4-di-O-benzyl-3-O-methyl-α-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With acetic acid In water Heating; | A n/a B 96% |
-
-
54189-41-2, 65378-12-3
MoO2Cl2(OP(N(CH3)2)3)2

-
-
4198-93-0
trityl hydroperoxide

-
A
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| In dichloromethane to Mo complex dissolved in CH2Cl2 added Ph3COOH, stirred for 30 min; concd., Et2O added; elem. anal.; | A 95% B 86% |


-
A
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In acetonitrile at 40℃; for 6h; Solvent; | A 94.1% B 94.2% |
-
-
596-43-0
bromo-triphenyl-methane

-
-
140-89-6
potassium ethyl xanthogenate

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
502-55-6
bis-ethoxythiocarbonyldisulfane

-
C
-
132277-76-0
O-ethyl S-triphenylmethyl dithiocarbonate

| Conditions | Yield |
|---|---|
| In benzene for 6h; Ambient temperature; | A 2% B 2% C 94% |

-
-
100675-64-7
Trityl-tert.butylaether

-
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| With water; acetic acid In dichloromethane for 0.25h; | 94% |
-
-
16928-73-7
triphenylmethyl phenyl sulfide

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In acetonitrile at -5℃; for 3h; Product distribution; Irradiation; photochemical reaction of sulfides with tetranitromethane; oxidation and fragmentation products; oxidation, fragmentation, deprotonation and aromatic substitution pathways; generation of radical cations by chemical oxidation with triarylaminium salts; | A 90% B 94% |
| With 5-methoxy-phenanthridinium In [D3]acetonitrile at 25℃; Irradiation; | A 27 % Spectr. B 13 % Spectr. |
| With N-methoxy phenanthridinium hexafluorophosphate In acetonitrile Photolysis; Inert atmosphere; | A 17.4 %Chromat. B 8.5 %Chromat. |
-
-
1246680-87-4
di-triphenylmethyl hyponitrite

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
4733-41-9
benzhydrylphenyl ether

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene In dichloromethane at 20℃; for 7h; Kinetics; | A 93.1% B 30.6% |
-
-
76-83-5
trityl chloride

-
-
6138-23-4, 138858-75-0
trehalose dihydrate

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
50705-44-7, 108811-30-9
6-O-(triphenylmethyl)-α-D-glucopyranosyl-6'-O-(triphenylmethyl)-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| In pyridine for 45h; Ambient temperature; | A n/a B 92% |

-
-
127136-68-9
C23H27N2O3P

-
-
76-84-6
triphenylmethyl alcohol

| Conditions | Yield |
|---|---|
| With sodium In ammonia | 92% |
| With ammonia; sodium In tetrahydrofuran for 0.25h; Product distribution; | 92% |
-
-
21504-18-7
2,2,2-Tris-methylsulfanyl-1-phenyl-ethanone

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
62575-83-1
triphenmethyl methyl sulfide

-
C
-
195305-56-7
Bis-methylsulfanyl-phenyl-thioacetic acid S-methyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane for 2h; Product distribution; Mechanism; Ambient temperature; various substrates under different reaction conditions; | A 23% B 72% C 92% |


| Conditions | Yield |
|---|---|
| With bismuth(III) chloride; silver(I) bromide; magnesium; copper(ll) bromide In tetrahydrofuran; toluene at 96℃; for 12h; Barbier Coupling Reaction; | 91% |
| With magnesium 1.) terbutyl methyl ether, THF, iodine, dichloroethane, reflux, 2.) reflux, 2.5 h; Multistep reaction; | |
| Stage #1: bromobenzene With magnesium In tetrahydrofuran at 55℃; for 0.333333h; Grignard reaction; Inert atmosphere; Sonication; Stage #2: benzophenone In tetrahydrofuran at 55℃; for 0.166667h; Grignard reaction; Sonication; | |
| Stage #1: bromobenzene With magnesium In diethyl ether at 35℃; for 2h; Stage #2: benzophenone In diethyl ether for 24h; Reflux; |

| Conditions | Yield |
|---|---|
| With tert-butylbenzene; tributylphosphine for 6.5h; Heating; | A 4% B 91% |
| With oxygen; N-hydroxyphthalimide In benzonitrile at 100℃; under 760 Torr; for 20h; | A 20 % Chromat. B 30 % Chromat. |
| With 3-tert-butylbenzene-1,2-diol; oxygen; Na11Zn3 W19Ru2O70H3 In 1,2-dichloro-ethane at 80℃; for 24h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With ozone In dichloromethane at 25℃; for 1h; Mechanism; Kinetics; Rate constant; other reaction times; | |
| With oxygen; Na11Zn3 W19Ru2O70H3 In 1,2-dichloro-ethane at 80℃; for 24h; Product distribution; also in the presence of tert-butylcatechol; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene at 23℃; for 4h; Solvent; Time; Inert atmosphere; Schlenk technique; | A 91% B n/a |

-
-
61040-98-0
3,6-diphenyl-1,2,4-trioxane

-
-
591-51-5
phenyllithium

-
A
-
93-56-1
phenylethane 1,2-diol

-
B
-
91-01-0
1,1-Diphenylmethanol

-
C
-
76-84-6
triphenylmethyl alcohol

-
D
-
100-51-6
benzyl alcohol

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 4.5h; Product distribution; Mechanism; reaction with alkylmagnesium galides, single electron transfer mechanism; | A 39% B 90% C 26% D 26% |

-
-
73080-19-0
O2,2'-anhydro-5,6-dihydro-6-(S)-(1,3-dithian-2-yl)-5'-O-trityluridine

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
73092-24-7
3-<(S)-1-(1,3-dithian-2-yl)>propionamido-(1,2-dideoxy-β-D-arabinofurano)-<1,2-d>-2-oxazolidinone

-
C
-
73080-26-9
5,6-dihydro-6-(S)-(1,3-dithian-2-yl)-1-β-D-arabinofuranosyluracil

| Conditions | Yield |
|---|---|
| With acetic acid for 10h; Heating; | A n/a B 90% C 10% |

-
-
82048-30-4
methyl 4-hydroxymethyl-1-triphenylmethylimidazole-5-carboxylate

-
A
-
76-84-6
triphenylmethyl alcohol

-
B
-
82032-43-7
methyl 4(5)-hydroxymethylimidazole-5(4)-carboxylate

| Conditions | Yield |
|---|---|
| In ethanol; acetic acid for 0.75h; Heating; | A n/a B 90% |

| Conditions | Yield |
|---|---|
| With hydridotetracarbonylcobalt In dichloromethane at 20℃; Rate constant; | 100% |
| With dimethylsilicon dichloride; sodium iodide In dichloromethane; acetone for 0.166667h; Ambient temperature; | 100% |
| With iodine; hypophosphorous acid In acetic acid at 60℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With Lawessons reagent In 1,2-dimethoxyethane for 15h; Mechanism; Ambient temperature; various alcohols, other solvents, other temperatures; | 100% |
| With Lawessons reagent In toluene for 0.2h; Quantum yield; Heating; DME, room temperature; | 100% |
| With Lawessons reagent In 1,2-dimethoxyethane for 15h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With Vilsmeier reagent In 1,4-dioxane at 80℃; for 0.5h; | 100% |
| With hydrogenchloride; calcium chloride In water; toluene at 25℃; for 5h; Solvent; Reagent/catalyst; | 96% |
| With chloro-trimethyl-silane In dichloromethane; water at 0℃; for 0.666667h; | 90% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
68-11-1
mercaptoacetic acid

-
-
34914-36-8
2-(triphenylmethylthio)ethanoic acid

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 20℃; for 0.5h; | 100% |
| With trifluoroacetic acid In chloroform at 20℃; for 1h; | 98% |
| With trifluoroacetic acid In chloroform at 20℃; for 3h; | 95% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
118488-56-5
Triphenyl-methanol anion

| Conditions | Yield |
|---|---|
| With potassium hydride; cryptand 222B In benzene for 0.0833333h; | 100% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
156-57-0
2-mercaptoethylamine hydrochloride

-
-
1095-85-8
2-amino tritylthio ethane

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 20℃; for 3h; | 100% |
| With boron trifluoride diethyl etherate; triethylamine In chloroform at 75℃; | 99% |
| With boron trifluoride diethyl etherate; triethylamine In chloroform at 75℃; | 99% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chloroform at 20℃; for 3.5h; Inert atmosphere; | 100% |
| With dodecylbenzene-sulphonic acid In water at 80℃; for 24h; | 97% |

| Conditions | Yield |
|---|---|
| In not given byproducts: (C6H5)3CH; rection of aluminum compd. with alcohol at -78°C; | 100% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
156-57-0
2-mercaptoethylamine hydrochloride

-
-
15297-43-5
<2-<(triphenylmethyl)thio>ethyl>amine hydrochloride

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| at 100℃; for 3h; Ionic liquid; Inert atmosphere; regioselective reaction; | 99% |
| With pentafluorophenylboronic acid In 1,2-dichloro-ethane for 16h; Friedel-Crafts arylation; Reflux; Molecular sieve; | 90% |
| With pentafluorophenylboronic acid In toluene for 2h; Molecular sieve; Reflux; | 70% |
| With acetic acid |

| Conditions | Yield |
|---|---|
| With pentafluorophenylboronic acid In 1,2-dichloro-ethane for 16h; Friedel-Crafts arylation; Reflux; Molecular sieve; | 99% |
| With zinc(II) chloride In 1,4-dioxane at 110 - 130℃; | 98% |
| With iodine In acetonitrile at 20℃; for 0.75h; | 98% |
| With phosphomolybdic acid In ethylenediamine at 60℃; for 1h; | 97% |
| With scandium tris(trifluoromethanesulfonate) In dichloromethane at 60℃; for 4h; Sealed tube; | 88% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
868-59-7, 7319-36-0, 75521-14-1, 93964-73-9
chlorhydrate du L-cysteinate d'ethyle

-
-
27486-86-8
S-trityl-L-cysteinate d'ethyle

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 0.25h; | 99% |

| Conditions | Yield |
|---|---|
| With Cp2Ti(OSO2C8F17)2 at 100℃; for 0.4h; Neat (no solvent); | 99% |
| Stage #1: triphenylmethyl alcohol; acetic anhydride With molybdenium(VI) dioxodichloride In toluene Stage #2: With N-ethyl-N,N-diisopropylamine In toluene for 2h; Heating; | 98% |
| K5 In acetonitrile at 20℃; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate In dichloromethane at 20℃; | 99% |
| With 3-dodecyl-2-iodo-1-methyl-1H-imidazol-3-ium hexafluoroantimonate; iodine In nitromethane at 20℃; for 2h; Inert atmosphere; | 99% |
| With titanium tetrachloride In dichloromethane at 20℃; for 0.0166667h; | 98% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 3h; Heating; | 99% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
1538-75-6
2,2-dimethylpropanoic anhydride

-
-
24523-65-7
2,2-dimethylpropionic acid trityl ester

| Conditions | Yield |
|---|---|
| Stage #1: triphenylmethyl alcohol; 2,2-dimethylpropanoic anhydride With molybdenium(VI) dioxodichloride In toluene Stage #2: With N-ethyl-N,N-diisopropylamine In toluene for 12h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With copper diacetate; chloro(1,5-cyclooctadiene)rhodium(I) dimer In o-xylene at 170℃; for 4h; | 99% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
7677-24-9
trimethylsilyl cyanide

-
-
6639-43-6
2,2,2-triphenylacetonitrile

| Conditions | Yield |
|---|---|
| With iodine; lithium carbonate In dichloromethane at 35℃; for 5h; | 99% |
| With tris(pentafluorophenyl)borate In acetonitrile at 20℃; for 1.16667h; | 93% |
| With tris(pentafluorophenyl)borate In acetonitrile at 20℃; for 17h; | 57% |
| With indium(III) chloride In toluene at 20℃; | 89 %Chromat. |
-
-
76-84-6
triphenylmethyl alcohol

-
-
1434816-93-9
2-chloro-4-fluoro-1-tritylsulfanylbenzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 1.5h; Cooling with ice; | 99% |
| With trifluoroacetic acid In dichloromethane at 20℃; for 1.5h; | 99% |
-
-
76-84-6
triphenylmethyl alcohol

-
-
156-57-0
2-mercaptoethylamine hydrochloride

-
-
76-05-1
trifluoroacetic acid

| Conditions | Yield |
|---|---|
| at 20℃; for 2h; | 98.5% |
| at 20℃; for 1h; |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 5h; Heating; | 98% |
| With sulfuric acid at 210 - 240℃; |

| Conditions | Yield |
|---|---|
| ammonium cerium(IV) nitrate for 0.25h; Heating; | 98% |
| With sulfuric acid for 4.5h; Ambient temperature; | 93% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone for 0.75h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: triphenylmethyl alcohol; l-cysteine hydrochloride With trifluoroacetic acid for 2h; Stage #2: With sodium acetate; sodium hydroxide In diethyl ether; water at 0℃; pH=5 - 6; | 98% |
| With trifluoroacetic acid for 3h; Inert atmosphere; | 96% |
| With choline chloride; urea In trifluoroacetic acid at 25℃; for 2h; | 95% |
Triphenylmethanol Specification
Triphenylmethanol is also known as Trityl alcohol, Triphenylcarbinol, 76-84-6, Methanol, triphenyl-, Tritanol, Triphenylmethyl alcohol, triphenylmethan-1-ol. With the Molecular Formula C19H16O, it is an organic compound and a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. It reacts with acetyl chloride to give triphenylmethyl chloride.
Physical properties about Triphenylmethanol are: (1)ACD/LogP: 3.836; (2)ACD/LogD (pH 5.5): 3.84; (3)ACD/LogD (pH 7.4): 3.84; (4)ACD/BCF (pH 5.5): 484.72; (5)ACD/BCF (pH 7.4): 484.71; (6)ACD/KOC (pH 5.5): 2909.88; (7)ACD/KOC (pH 7.4): 2909.86; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.622; (12)Molar Refractivity: 81.072 cm3; (13)Molar Volume: 230.289 cm3; (14)Polarizability: 32.14 10-24cm3; (15)Surface Tension: 46.4389991760254 dyne/cm; (16)Density: 1.13 g/cm3; (17)Flash Point: 168.744 °C; (18)Enthalpy of Vaporization: 66.255 kJ/mol; (19)Boiling Point: 379.999 °C at 760 mmHg
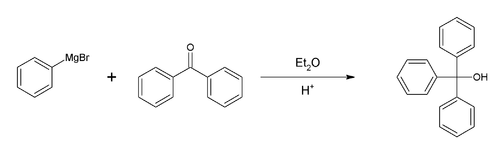
Preparation of Triphenylmethanol: The preparation of triphenylmethanol from methyl benzoate or benzophenone and bromobenzene is a common laboratory experiment for teaching the Grignard reaction. An alternative starting material is diethyl carbonate.
When you are using this chemical, please be cautious about it as the following:
1. Do not breathe dust;
2. Avoid contact with skin and eyes;
3. Wear suitable protective clothing;
4. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
You can still convert the following datas into molecular structure:
(1)InChI=1S/C19H16O/c20-19(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15,20H;
(2)InChIKey=LZTRCELOJRDYMQ-UHFFFAOYSA-N;
(3)SmilesC(c1ccccc1)(c1ccccc1)(c1ccccc1)O;
Related Products
- Triphenylmethanol
- 76848-19-6
- 768-48-9
- 768-52-5
- 7685-44-1
- 76855-69-1
- 768-56-9
- 76857-14-2
- 76858-94-1
- 768-59-2
- 768-60-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View