-
Name
Uridine Monophosphate
- EINECS 200-408-0
- CAS No. 58-97-9
- Article Data126
- CAS DataBase
- Density 1.865 g/cm3
- Solubility
- Melting Point 202°C (rough estimate)
- Formula C9H13 N2 O9 P
- Boiling Point °Cat760mmHg
- Molecular Weight 324.184
- Flash Point °C
- Transport Information
- Appearance
- Safety Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic vapors of NOx and POx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 5'-Monophosphate, Uridine;Acid, Uridylic;Acids, Uridylic;Monophosphate, Uridine;UMP;Uridine 5' Monophosphate;Uridine 5'-Monophosphate;Uridine Monophosphate;Uridylic Acid;Uridylic Acids
- PSA 181.12000
- LogP -2.73490
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine; benzenesulfonamide for 0.5h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: uridine With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene; trichlorophosphate at 20℃; for 0.25h; Flow reactor; Green chemistry; Stage #2: With water at 20℃; Temperature; Flow reactor; Green chemistry; chemoselective reaction; | 89% |
| With tris(p-nitrophenyl)phosphate at 50℃; Erwina herbicola 47/3 cells; | 78% |
| With pyridine; trichlorophosphate |
-
-
56428-57-0
uridine 5’-monophosphate-imidazole

-
A
-
58-97-9
5'-Uridylic Acid

-
B
-
3803-29-0
[5']uridylic acid monofluoride

| Conditions | Yield |
|---|---|
| With water; lithium fluoride at 25℃; pH=8.6; Reagent/catalyst; pH-value; | A 18.7% B 76.3% |
-
-
58-96-8
uridine

-
A
-
58-97-9
5'-Uridylic Acid

-
B
-
15548-52-4
O-((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl O,O-dihydrogen phosphorothioate

| Conditions | Yield |
|---|---|
| With trichlorothiophosphine In various solvent(s) for 92h; Ambient temperature; Title compound not separated from byproducts; | A 14.5% B 60.9% |

| Conditions | Yield |
|---|---|
| Stage #1: uridine With N,N,N',N'-tetramethyl-1,8-diaminonaphthalene; trichlorophosphate In dimethyl sulfoxide at 20℃; for 0.25h; Flow reactor; Green chemistry; Stage #2: With water In dimethyl sulfoxide at 20℃; Reagent/catalyst; Flow reactor; Green chemistry; | A 29% B 36% |
-
-
15715-58-9
triethylamine carbonate

-
-
27908-36-7
uridine 5'-monophosphate morpholidate

-
B
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| Stage #1: fluoromethylenebis(phosphonic acid) tri-n-butylammonium salt With tributyl-amine In ethanol; water at 20℃; for 0.5h; pH=4.5; Stage #2: triethylamine carbonate; uridine 5'-monophosphate morpholidate In dimethyl sulfoxide at 20℃; for 72h; pH=7; Inert atmosphere; | A 20% B n/a |


-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With isopentyl nitrite In acetone for 24h; | 15% |

| Conditions | Yield |
|---|---|
| With pyridine; trichlorophosphate at -35 - -30℃; anschl. Erwaermen mit wss. H2SO4; | |
| With pyrophosphoryl chloride anfangs bei -30grad, und Hydrolysieren des Rktprod. mit Hilfe von LiOH; | |
| With pyridine; chlorophosphoric acid dibenzyl ester at -40 - -30℃; anschl. Hydrierung; |

| Conditions | Yield |
|---|---|
| Biosynthese; |
-
-
3257-71-4, 26302-00-1, 51096-52-7, 51096-53-8
(R)-2',3'-O-benzylideneuridine

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With pyridine; chlorophosphoric acid dibenzyl ester at -15℃; anschl. Hydrierung; |
-
-
97-55-2, 7540-64-9, 99945-37-6, 130384-52-0
5-phosphoribosyl-1-pyrophosphate

-
-
66-22-8
uracil

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| reversible enzymatische Bildung mit Hilfe von Uracil-phosphoribosyltransferase aus Tumor-Extrakten; | |
| reversible enzymatische Bildung mit Hilfe von Uracil-phosphoribosyltransferase aus Lactobacillus-Arten; | |
| reversible enzymatische Bildung mit Hilfe von Uracil-phosphoribosyltransferase aus Escherichia coli-Staemmen; | |
| With uracil phosphoribosyltransferase from Thermus thermophilus HB8; magnesium chloride In aq. buffer at 60℃; pH=8; Kinetics; Enzymatic reaction; |

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With ethanol; platinum Hydrogenation; |

| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; Equilibrium constant; pH 8.2-8.8; |


| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; pH 8.8; |

-
-
4004-57-3
uridine 3',5'-cyclic monophosphate

-
A
-
84-53-7
uridine-3'-phosphate

-
B
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| Lactuca phosphodiesterase In water for 0.416667h; Product distribution; Tris-HCl buffer (pH 6.5); effect of purification stage of phosphodiesterase; |
-
-
4004-57-3
uridine 3',5'-cyclic monophosphate

-
A
-
84-53-7
uridine-3'-phosphate

-
B
-
58-97-9
5'-Uridylic Acid

-
C
-
131-83-9
uridine 2'-monophosphate

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 90.1℃; Rate constant; |

-
-
83416-85-7
Phosphoric acid bis-(5-chloro-quinolin-8-yl) ester (2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-3-hydroxy-4-(tetrahydro-pyran-2-yloxy)-tetrahydro-furan-2-ylmethyl ester

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc(II) chloride 1.) pyridine, water, room temp., 24 h, 2.) 22 deg C, 20 h; Yield given. Multistep reaction; |
-
-
99564-44-0
uridine 5'-(β-D-glucopyranosyl phosphoric acid)

-
A
-
50-99-7
D-glucose

-
B
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 25℃; for 40h; |
-
-
55728-64-8
O2',O3'-isopropylidene-[5']uridylic acid

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 25℃; for 12h; Yield given; | |
| With DOWEX(R) 50WX2-200 |

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 2h; |
-
-
19140-04-6
1-(2,3-O-ethoxymethylidene-β-D-ribofuranosyl)uracil

-
A
-
58-97-9
5'-Uridylic Acid

-
B
-
58-96-8
uridine

-
C
-
15548-52-4
O-((2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl O,O-dihydrogen phosphorothioate

| Conditions | Yield |
|---|---|
| With 1H-imidazole; trichlorophosphate 1.) acetonitrile, room temp. 3 days; 2.) 50percent CH3COOH, 50 deg C, 1 h.; Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | |
| With 1H-imidazole; trichlorophosphate 1.) acetonitrile, room temp. 3 days; 2.) 50percent CH3COOH, 50 deg C, 1 h.; Yield given. Multistep reaction. Title compound not separated from byproducts; |


-
A
-
606-02-0
uridine 2',3'-cyclic phosphate

-
B
-
84-53-7
uridine-3'-phosphate

-
C
-
58-97-9
5'-Uridylic Acid

-
D
-
66-22-8
uracil

| Conditions | Yield |
|---|---|
| With sodium persulfate; buffer pH=7 at 70℃; for 4h; Further byproducts given; | A 4 % Chromat. B 25 % Chromat. C 8 % Chromat. D 48 % Chromat. |


-
A
-
84-53-7
uridine-3'-phosphate

-
B
-
58-97-9
5'-Uridylic Acid

-
C
-
131-83-9
uridine 2'-monophosphate

-
D
-
66-22-8
uracil

| Conditions | Yield |
|---|---|
| With sodium persulfate; buffer pH=7 at 70℃; for 4h; Further byproducts given; | A 25 % Chromat. B 8 % Chromat. C 43 % Chromat. D 48 % Chromat. |
| With sodium persulfate at 70℃; for 4h; Further byproducts given; | A 25 % Chromat. B 8 % Chromat. C 43 % Chromat. D 48 % Chromat. |


-
A
-
606-02-0
uridine 2',3'-cyclic phosphate

-
B
-
84-53-7
uridine-3'-phosphate

-
C
-
58-97-9
5'-Uridylic Acid

-
D
-
131-83-9
uridine 2'-monophosphate

-
E
-
66-22-8
uracil

| Conditions | Yield |
|---|---|
| With sodium persulfate; sodium phosphate buffer pH=7 In water at 70℃; for 4h; Product distribution; other compounds; | A 1 % Chromat. B 33 % Chromat. C 19 % Chromat. D 2 % Chromat. E 44 % Chromat. |


-
A
-
606-02-0
uridine 2',3'-cyclic phosphate

-
B
-
84-53-7
uridine-3'-phosphate

-
C
-
58-97-9
5'-Uridylic Acid

-
D
-
66-22-8
uracil

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; buffer pH=7 at 70℃; for 4h; Further byproducts given; | A 1 % Chromat. B 33 % Chromat. C 19 % Chromat. D 44 % Chromat. |


-
A
-
84-53-7
uridine-3'-phosphate

-
B
-
58-97-9
5'-Uridylic Acid

-
C
-
131-83-9
uridine 2'-monophosphate

-
D
-
66-22-8
uracil

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; buffer pH=7 at 70℃; for 4h; Further byproducts given; | A 33 % Chromat. B 19 % Chromat. C 2 % Chromat. D 44 % Chromat. |


-
A
-
58-97-9
5'-Uridylic Acid

-
B
-
61-19-8
5'-adenosine monophosphate

-
C
-
85-32-5
5'-guanosine monophosphate

| Conditions | Yield |
|---|---|
| With SVP (1 unit) In water at 37℃; for 12h; Product distribution; HPLC analysis; no reaction with RNaseT2 or spleen phosphodiesterase; |


| Conditions | Yield |
|---|---|
| With SVP (1 unit) In water at 37℃; for 12h; Product distribution; HPLC analysis; no reaction with RNaseT2 or spleen phosphodiesterase; |

| Conditions | Yield |
|---|---|
| With water at 90.1℃; Rate constant; var. pH; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 50℃; for 33h; relative half-life; further hydrolytic conditions: with ribonuclease A, with snake venom phosphodiesterase; further reaction time and temperature; |
-
-
58-97-9
5'-Uridylic Acid

-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
65062-80-8
O2',O3'-carbonyl-O5'-(hydroxy-imidazol-1-yl-phosphoryl)-uridine

| Conditions | Yield |
|---|---|
| In acetonitrile for 1h; Milling; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole; 5'-Uridylic Acid With 2,2'-dipyridyldisulphide; triethylamine; triphenylphosphine In dimethyl sulfoxide; N,N-dimethyl-formamide for 3h; Stage #2: With sodium perchlorate; triethylamine In diethyl ether; dimethyl sulfoxide; N,N-dimethyl-formamide; acetone for 2h; | 85% |
| Stage #1: 1H-imidazole; 5'-Uridylic Acid With 2,2'-dipyridyldisulphide; triethylamine; triphenylphosphine In dimethyl sulfoxide; N,N-dimethyl-formamide Stage #2: With sodium perchlorate; triethylamine In diethyl ether; dimethyl sulfoxide; N,N-dimethyl-formamide; acetone for 2h; | |
| Stage #1: 1H-imidazole; 5'-Uridylic Acid With 2,2'-dipyridyldisulphide; triethylamine; triphenylphosphine In dimethyl sulfoxide; N,N-dimethyl-formamide for 2h; Stage #2: With sodium perchlorate; triethylamine In diethyl ether; dimethyl sulfoxide; N,N-dimethyl-formamide; acetone for 0.5h; |
-
-
807318-50-9
(1S,2R,3S,4R,5R)-2,3,4-trihydroxy-5-(hydroxymethyl)cyclohexyl dihydrogen phosphate

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With magnesium chloride hexahydrate; acetylphosphoric acid; acetate kinase; glucose-1-phosphate uridyltransferase; UMP kinase; tris hydrochloride; ATP In water at 37℃; for 5h; Enzymatic reaction; | 84.4% |
-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 2068.65 Torr; for 24h; | 83% |

| Conditions | Yield |
|---|---|
| With calcium chloride In N,N-dimethyl acetamide Reagent/catalyst; Solvent; | 82% |

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| Stage #1: diimidazolylpyrophosphate; 5'-Uridylic Acid With calcium chloride In N,N-dimethyl-formamide at 30℃; for 4h; Cooling with ice; Stage #2: With hydrogenchloride; sodium chloride In water Reagent/catalyst; | 80% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water for 6h; pH 5; | 79.1% |
-
-
19191-91-4
1,2-dioctanoyl-sn-glycero-3-phosphocholine

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| phospholipase D-P In chloroform at 40℃; for 6h; | 79% |
-
-
636-70-4
triethylamine hydrobromide

-
-
58-97-9
5'-Uridylic Acid

-
-
1356191-61-1
5-iodouridine-5'-monophosphate triethylammonium

| Conditions | Yield |
|---|---|
| Stage #1: 5'-Uridylic Acid With iodine; nitric acid In chloroform; water at 80℃; for 12h; Stage #2: triethylamine hydrobromide | 77% |

| Conditions | Yield |
|---|---|
| With calcium chloride In dimethyl sulfoxide Reagent/catalyst; Solvent; | 76% |
-
-
58-97-9
5'-Uridylic Acid

-
-
21016-94-4, 7531-46-6
5-iodouridine-5’-monophosphate

| Conditions | Yield |
|---|---|
| With iodine; nitric acid In chloroform at 90℃; for 18h; | 75% |
| With iodine; nitric acid In chloroform at 80℃; for 12h; Product distribution / selectivity; |
-
-
58-97-9
5'-Uridylic Acid

-
-
76742-17-1
cytidine 5'-monophosphate morpholidate

| Conditions | Yield |
|---|---|
| With 4,5-dicyano-1H-imidazole In N,N-dimethyl-formamide at 20℃; for 24h; Reagent/catalyst; Time; Concentration; Temperature; | 68% |
-
-
58-97-9
5'-Uridylic Acid

-
-
120138-94-5
C9H14N2O11P2

| Conditions | Yield |
|---|---|
| With sodium hypophosphate at 60℃; pH 6; | 66% |
-
-
58-97-9
5'-Uridylic Acid

-
-
405218-94-2
2-amino-3,4-di-O-acetyl-2-deoxy-α-D-fucopyranosyl phosphate

| Conditions | Yield |
|---|---|
| Stage #1: 5'-Uridylic Acid With tributyl-amine In 1,4-dioxane for 0.25h; Stage #2: With 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 3h; Stage #3: 2-amino-3,4-di-O-acetyl-2-deoxy-α-D-fucopyranosyl phosphate In N,N-dimethyl-formamide at 20℃; for 24h; | 47% |

| Conditions | Yield |
|---|---|
| With lithium chloride In water at 85℃; for 8h; pH=Ca. 2.5; Reagent/catalyst; Inert atmosphere; | 42% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 5h; Ambient temperature; | 38% |

| Conditions | Yield |
|---|---|
| With N-ethylmorpholine buffer; phosphorotriimidazolide at 22℃; for 72h; other 5'-nucleotides, also with phosphorotribenzimidazolide, MnCl2; other time, temperature; | A 25% B 36% |
| With phosphorotriimidazolide; magnesium chloride at 22℃; for 72h; N-ethylmorpholine buffer (pH 7.0); | A 25% B 36% |
-
-
88949-01-3
C18H23N5O5

-
-
58-97-9
5'-Uridylic Acid

-
-
132019-16-0
2-amino-7-(dimethylamidino)mitosene 1-uridylate uridylic acid

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 16h; Ambient temperature; | 32% |

-
-
58-97-9
5'-Uridylic Acid

| Conditions | Yield |
|---|---|
| With tributyl-amine In water; sodium hydrogencarbonate; N,N-dimethyl-formamide | 30% |

| Conditions | Yield |
|---|---|
| Stage #1: 5'-Uridylic Acid; 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide at 20℃; for 3h; Stage #2: α-D-galactofuranosyl bis(triethylammonium)phosphate salt In N,N-dimethyl-formamide at 20℃; for 19h; | 26% |

-
-
58-97-9
5'-Uridylic Acid

-
-
4055-39-4
mitomycin A

-
-
132019-12-6
2-amino-7-methoxymitosene 1-uridylate uridilic acid salt

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 3h; Ambient temperature; | 25% |
-
-
1151903-36-4
(1S,2R,3R,4R,5R)-2-acetamido-3,4-dihydroxy-5-hydroxymethyl-cyclohexane-1-phosphate

-
-
58-97-9
5'-Uridylic Acid

-
-
1151903-27-3
uridine 5'-(5a-carba-α-D-N-acetylglucosaminopyranosyl diphosphate)

| Conditions | Yield |
|---|---|
| With magnesium chloride hexahydrate; acetylphosphoric acid; acetate kinase; GlcNAc-1-phosphate uridyltransferase; UMP kinase; tris hydrochloride; ATP In water at 37℃; for 5h; Enzymatic reaction; | 23% |
-
-
4715-05-3
3,4,6-tri-O-acetyl-α-D-glucopyranose-1,2-(t-butylorthoacetat)

-
-
58-97-9
5'-Uridylic Acid

-
-
81788-35-4
uridine 5'-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl phosphoric acid)

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 20h; | 21% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 50 - 60℃; | 21% |
-
-
58-97-9
5'-Uridylic Acid

-
-
70343-57-6
C21H22N4O6

-
-
132019-14-8
2-amino-7-(4-hydroxyanilino)mitosene 1-uridilate uridylic acid salt

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 3h; Ambient temperature; | 21% |
Uridylic acid Chemical Properties
IUPAC Name: [(2R,3S,4R,5R)-5-(2,4-Dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate
Synonyms: Uridine 5′-monophosphate ; Uridine-5'-monophosphate ; Uridine-5'-phosphate ; Uridine-monophosphate ; Uridine-phosphate ; Uridylate ; Uridylic acid
The Molecular Formula of Uridylic acid (58-97-9):C9H13N2O9P
The Molecular Weight of Uridylic acid (58-97-9):324.1813 g/mol
The Molecular Structure of Uridylic acid (58-97-9):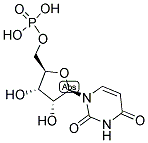
Index of Refraction: 1.643
Molar Refractivity: 62.88 cm3
Molar Volume: 173.8 cm3
Polarizability: 10-24cm3
Surface Tension: 104.6 dyne/cm
Density: 1.865 g/cm3
Uridylic acid Uses
1. Uridylic acid (58-97-9) can be used as intermediate of nucleic acid drug.
2.It can also be used as biochemical reagent.
Uridylic acid Toxicity Data With Reference
| 1. | ipr-mus LD :>1 g/kg | ANYAA9 Annals of the New York Academy of Sciences. 60 (1954),251. |
Uridylic acid Safety Profile
Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic vapors of NOx and POx.
WGK Germany: 3
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View