-
Name
beta-Carotene
- EINECS 230-636-6
- CAS No. 7235-40-7
- Article Data41
- CAS DataBase
- Density 0.941 g/cm3
- Solubility 100 μg/mL in hexane
- Melting Point 176-184 °C (dec.)
- Formula C40H56
- Boiling Point 654.7 °C at 760 mmHg
- Molecular Weight 536.885
- Flash Point 346 °C
- Transport Information
- Appearance red to purple power
- Safety 7-15-18-36-26
- Risk Codes 44-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Provitamin A;beta Carotene;CPD1F-129;Cyclohexene, 1,1-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethyl-, (all-E)-;trans-B-Carotene;KPMK;Betacarotenum [Latin];(1E,3Z,5E,7Z,9E,11E,13E,15E)-3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,5,7,9,11,13,15,17-nonaene;(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,5,7,9,11,13,15,17-nonaene;a,a-Carotene;Cyclohexene, 1,1-(3,7,12,16-tetramethyl-1,3,5, 7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6,6-trimethyl-, (all-E)-;all-trans-.beta.-Carotene;Solatene (caps);beta-Karotin;Cyclohexene, 1,1-(3,7,12,16-tetramethyl-1,3,5, {7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6,6-trimethyl-,} (all-E)-;Zlut prirodni 26 [Czech];Lucarotin;Provatene;(all-E)-1,1-(3,7,12,16-Tetramethyl-1,3,5, 7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6, 6-trimethylcyclohexene];Food orange 5;Betacaroteno [Spanish];all-trans-beta-Carotene;.beta.-Carotene, all-trans-;1,1-(3,7,12,16-Tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethylcyclohexene), (all E)-;Karotin [Czech];31797-85-0;(all-E)-1,1-(3,7,12,16-Tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethylcyclohexene);Solatene;Betacaroteno [INN-Spanish];b-Carotene;Diet,beta-carotene supplementation;beta,beta-Carotene;3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,5,7,9,11,13,15,17-nonaene;Carotaben;beta-Carotene, all-trans-;β-Carotene;B-Carotene; Carotin; Provitanin A;Beta- Carotene Beta;natracol beta carotene wsp1;beta-Carotene natural;
- PSA 0.00000
- LogP 12.60580
Synthetic route
-
-
5056-17-7
(2E,4E,6E)-2,7-dimethyl-2,4,6-octatrienedial

-
-
59057-30-6
(1E)-1-(2,6,6-trimethylcyclohex-1-enyl)-3-methyl-1,4-pentadien-3-ol

-
-
603-35-0
triphenylphosphine

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: (1E)-1-(2,6,6-trimethylcyclohex-1-enyl)-3-methyl-1,4-pentadien-3-ol; triphenylphosphine With hydrogenchloride In methanol; water at 45℃; for 1h; Stage #2: (2E,4E,6E)-2,7-dimethyl-2,4,6-octatrienedial With 1,2-bis (3-methylimidazolium-1-yl) ethane dihydroxide; sodium carbonate In methanol; water at 20℃; for 3h; Reagent/catalyst; Temperature; Wittig Olefination; Inert atmosphere; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: Retinol acetate With sulfuric acid; triphenylphosphine In methanol at 0 - 5℃; for 10.5h; Stage #2: With palladium diacetate; β‐cyclodextrin In ethanol; dichloromethane; water under 16501.7 Torr; for 8h; Reagent/catalyst; Solvent; Pressure; Temperature; Autoclave; | 90.6% |
| Stage #1: Retinol acetate With aniline In ethanol Stage #2: With hydrogenchloride; triphenylphosphine In ethanol; water at 10 - 25℃; for 26h; Reagent/catalyst; Solvent; Further stages; | 58.75% |

-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: C35H48Si With tetrabutyl ammonium fluoride In tetrahydrofuran at 0℃; for 0.5h; Inert atmosphere; Stage #2: 2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene With tris(dibenzylideneacetone)dipalladium(0) chloroform complex In tetrahydrofuran at 0 - 25℃; for 1.25h; Inert atmosphere; | 90% |
-
-
1427286-50-7
(2E,4E,6E,8E,10E,12E,14E)-2,15-diiodo-6,11-dimethylhexadeca-2,4,6,8,10,12,14-heptaene

-
-
1427286-52-9, 791131-03-8
tributyl [(E)-2-(2,6,6-trimethylcyclohex-1-en-1-yl)ethenyl]stannane

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: (2E,4E,6E,8E,10E,12E,14E)-2,15-diiodo-6,11-dimethylhexadeca-2,4,6,8,10,12,14-heptaene; tributyl [(E)-2-(2,6,6-trimethylcyclohex-1-en-1-yl)ethenyl]stannane With tetrakis(triphenylphosphine) palladium(0); copper(I) thiophene-2-carboxylate; Tetrabutylammoniumsalz der Diphenylphosphinsaeure In N,N-dimethyl-formamide at 25℃; Stille Cross Coupling; Inert atmosphere; Stage #2: With 2,6-di-tert-butyl-4-methyl-phenol In diethyl ether; N,N-dimethyl-formamide; toluene at 25℃; Inert atmosphere; | 86% |
-
-
870636-68-3
5,14-bis(benzenesulfonyl)-3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,9,15,17-pentaene-6,13-diol bis(methoxymethyl) ether

-
A
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 80℃; for 16h; | A 84% B n/a |
-
-
870636-68-3
5,14-bis(benzenesulfonyl)-3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,9,15,17-pentaene-6,13-diol bis(methoxymethyl) ether

-
A
-
7235-40-7
beta-carotene

-
B
-
18526-49-3
(11Z)-β,β-carotene

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 80℃; for 16h; Product distribution / selectivity; | A 84% B n/a |

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane; benzene at 70 - 80℃; for 18h; | 81% |
| With potassium methanolate In cyclohexane; benzene at 70 - 80℃; for 18h; Product distribution / selectivity; | 81% |
-
-
251291-82-4
11,20-dibenzenesulfonyl-11,12,19,20-tetrahydro-β-carotene

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With sodium In ethanol for 10h; desulfonation; Heating; | 75% |
| With sodium In ethanol for 10h; Heating / reflux; | 75% |

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With sodium In ethanol; toluene | 75% |
-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol; N-ethyl-N,N-diisopropylamine; bis(benzonitrile)palladium(II) dichloride In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 16h; Stille cross-coupling; | 73% |
-
-
1427286-50-7
(2E,4E,6E,8E,10E,12E,14E)-2,15-diiodo-6,11-dimethylhexadeca-2,4,6,8,10,12,14-heptaene

-
-
256518-16-8
(E)-4,4,5,5-tetramethyl-2-(2-(2,6,6-trimethylcyclohex-1-enyl)-vinyl)-1,3,2-dioxaborolane

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: (2E,4E,6E,8E,10E,12E,14E)-2,15-diiodo-6,11-dimethylhexadeca-2,4,6,8,10,12,14-heptaene With tetrakis(triphenylphosphine) palladium(0); 2,6-di-tert-butyl-4-methyl-phenol In tetrahydrofuran; toluene at 25℃; for 0.0833333h; Suzuki Coupling; Inert atmosphere; Stage #2: (E)-4,4,5,5-tetramethyl-2-(2-(2,6,6-trimethylcyclohex-1-enyl)-vinyl)-1,3,2-dioxaborolane With thallium(I) hydroxide In tetrahydrofuran; water; toluene at 25℃; for 4h; Suzuki Coupling; Inert atmosphere; | 73% |

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane; benzene at 70 - 80℃; for 18h; | 71% |
| With potassium methanolate In cyclohexane; benzene at 70 - 80℃; for 18h; Product distribution / selectivity; | 71% |
-
-
870636-67-2
5,14-bis(benzenesulfonyl)-3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,9,15,17-pentaene-6,13-diol bis(1-ethoxyethyl) ether

-
A
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 80℃; for 16h; | A 70% B n/a |

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane; benzene at 70 - 80℃; for 17h; | 70% |
| With potassium methanolate In cyclohexane; benzene at 70 - 80℃; for 17h; Product distribution / selectivity; | 70% |

| Conditions | Yield |
|---|---|
| With potassium methanolate In cyclohexane at 80℃; for 16h; Product distribution / selectivity; | A 70% B n/a |
-
-
56798-08-4
(E)-1-bromo-2-iodoethylene

-
-
75-24-1
trimethylaluminum

-
-
111917-89-6
1-((E,E,E)-3-methyl-1,3,5-octatrien-7-ynyl)-2,6,6-trimethyl-1-cyclohexene

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: trimethylaluminum; 1-((E,E,E)-3-methyl-1,3,5-octatrien-7-ynyl)-2,6,6-trimethyl-1-cyclohexene With zirconocene dichloride In dichloromethane at 23℃; for 4h; Stage #2: at 50℃; Stage #3: (E)-1-bromo-2-iodoethylene With tris(dibenzylideneacetone)dipalladium (0); zinc(II) chloride In tetrahydrofuran; N,N-dimethyl-formamide at 23℃; for 8h; Further stages.; | 68% |
| Stage #1: trimethylaluminum; 1-((E,E,E)-3-methyl-1,3,5-octatrien-7-ynyl)-2,6,6-trimethyl-1-cyclohexene; zirconocene dichloride In 1,2-dichloro-ethane at 20℃; for 20h; Stage #2: (E)-1-bromo-2-iodoethylene With zinc(II) chloride; tris(dibenzylideneacetone)dipalladium (0) In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 5h; Further stages.; | 68% |


| Conditions | Yield |
|---|---|
| Stage #1: C38H44ClP With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Stage #2: (R)-9-cis-13,14-dihydro-retinal In tetrahydrofuran; hexane at -78 - 25℃; for 1.5h; | 67% |

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium carbonate In water at 15℃; for 1h; | 66.1% |
| With bis(acetylacetonate)oxovanadium; 18-crown-6 ether; oxygen; potassium carbonate In toluene at 40℃; for 6h; Catalytic behavior; Concentration; Reagent/catalyst; Molecular sieve; Green chemistry; |

-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: C23H34OSi2 With potassium trimethylsilonate In tetrahydrofuran; 1,4-dioxane at 25℃; for 0.25h; Inert atmosphere; Stage #2: 2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene With bis(dibenzylideneacetone)-palladium(0) In 1,4-dioxane at 25℃; for 1.5h; Inert atmosphere; Stage #3: C23H34OSi2 Further stages; | 66% |
-
-
5056-17-7
(2E,4E,6E)-2,7-dimethyl-2,4,6-octatrienedial

-
-
3917-39-3
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexenyl)-2,4-pentadien-1-ol

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With tributylphosphine; phenyl isocyanate; tetrakis(triphenylphosphine) palladium(0) In acetonitrile for 5h; Heating; | 65% |

| Conditions | Yield |
|---|---|
| Stage #1: RETINOL With pyridine In methanol Stage #2: With hydrogen bromide; triphenylphosphine In methanol at 5 - 35℃; for 35h; Reagent/catalyst; Solvent; Further stages; | 64.42% |
| Multi-step reaction with 2 steps 1.1: hydrogenchloride / methanol; 1,4-dioxane / 2 h / 25 °C 2.1: n-butyllithium / tetrahydrofuran; hexane / 0.5 h / -78 °C 2.2: 1.5 h / -78 - 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| With Hoveyda-Grubbs catalyst second generation In toluene at 25℃; for 4.5h; Reagent/catalyst; | A 16% B 62% C 1% |


-
-
186194-14-9
diethyl 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienylphosphonate

-
-
5056-17-7
(2E,4E,6E)-2,7-dimethyl-2,4,6-octatrienedial

-
B
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran | A 61% B n/a |


-
-
138846-06-7
2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene

-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| Stage #1: (1E,3E,5E,7E,9E)-1,10-di(benzyldimethylsilyl)-3,8-dimethyldeca-1,3,5,7,9-pentaene With tetrabutyl ammonium fluoride In tetrahydrofuran at 0℃; for 0.666667h; Inert atmosphere; Stage #2: 2-[(1’E,3’E)-3’-methyl-4’-iodobuta-1’,3’-dien-1’yl]-1,3,3-trimethylcyclohex-1-ene With tris(dibenzylideneacetone)dipalladium(0) chloroform complex In tetrahydrofuran at 0 - 25℃; for 1.25h; Inert atmosphere; | 61% |
-
-
870636-64-9
5,14-bis(benzenesulfonyl)-6,13-dibromo-3,7,12,16-tetramethyl-1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)octadeca-1,3,9,15,17-pentaene

-
A
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In cyclohexane at 80℃; for 16h; | A 60% B n/a |

-
A
-
7235-40-7
beta-carotene

-
B
-
69774-12-5
methyl (2E,4E,6E,8E,10E,12E,14E)-4,9,13-trimethyl-15-(2,6,6-trimethylcyclohex-1-en-1-yl)pentadeca-2,4,6,8,10,12,14-heptaenoate

| Conditions | Yield |
|---|---|
| With Hoveyda-Grubbs catalyst second generation In toluene at 25℃; for 7h; | A 58% B 24% |


-
A
-
22255-47-6
(E)-2-(3-methylbuta-1,3-dienyl)-1,3,3-trimethyl-cyclohex-1-ene

-
B
-
7235-40-7
beta-carotene

-
C
-
43219-55-2
(1E,3E)-3-methyl-1-[2,6,6-trimethylcyclohex-1-enyl]hexa-1,3,5-triene

-
D
-
70244-78-9, 94440-28-5
2-((1E,3E,5E)-3,7-dimethylocta-1,3,5,7-tetraen-1-yl)-1,3,3-trimethylcyclohex-1-ene

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 50℃; for 6.5h; Inert atmosphere; | A 0.1% B 57% C 3% D 5% |


-
-
7235-40-7
beta-carotene

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium hydroxide In water at 15℃; for 2h; | 53.5% |

| Conditions | Yield |
|---|---|
| With cerium(IV) oxide; sulfuric acid; potassium iodide In dichloromethane; water at 0℃; under 760.051 Torr; for 2.5h; Reagent/catalyst; Temperature; UV-irradiation; | 99.5% |
| With sulfuric acid; copper(II) sulfate; potassium iodide In dichloromethane; water at -5 - 45℃; under 760.051 Torr; for 4h; Temperature; Reagent/catalyst; Green chemistry; | 98.05% |
| With dihydrogen peroxide; potassium iodide; lithium hydroxide In dichloromethane; water at -5 - 45℃; under 760.051 Torr; for 5.5h; Catalytic behavior; Temperature; Reagent/catalyst; Green chemistry; | 98.05% |

| Conditions | Yield |
|---|---|
| With oxygen In water under 760.051 Torr; for 8h; Solvent; Reagent/catalyst; | 89% |
| With osmium(VIII) oxide; diethyl ether; sodium sulfate Reagens 4: wss. H2O2; | |
| With chloroform; dihydrogen peroxide; acetic acid | |
| With manganese(IV) oxide |
beta-Carotene Chemical Properties
Structure of beta-Carotene (CAS NO.7235-40-7):
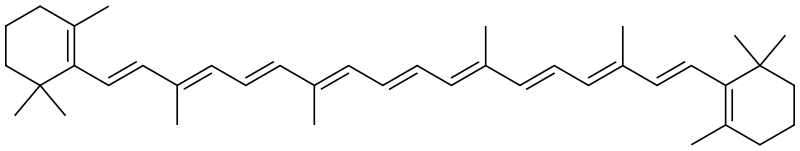
Empirical Formula: C40H56
Molecular Weight: 536.8726g/mol
IUPAC Name: 1,3,3-trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene
EINECS: 230-636-6
Index of Refraction: 1.565
Molar Refractivity: 185.93 cm3
Molar Volume: 570.1 cm3
Polarizability: 73.71×10-24cm3
Surface Tension: 36.3 dyne/cm
Density: 0.941 g/cm3
Flash Point: 346 °C
Enthalpy of Vaporization: 92.93 kJ/mol
Melting Point: 176-184 °C (dec.)
Boiling Point: 654.7 °C at 760 mmHg
Vapour Pressure: 2.71E-16 mmHg at 25°C
Solubility hexane: 100 μg/mL, soluble
Stability: Stable, but sensitive to air, heat and light. Store at -20C under nitrogen. Pyrophoric - may ignite spontaneously in air at room temperature.
Product Categories: Organics;Antioxidant;Biochemistry;Vitamins;Vitamins and derivatives;Intermediates & Fine Chemicals;Pharmaceuticals;Retinoids;Nutraceuticals;Vitamin Ingredients;Nutritional Ingredients
Canonical SMILES: CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC=CC=C(C)C=CC=C(C)C=CC2=C(CCCC2(C)C)C)C)C
Isomeric SMILES: CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C/C=C/C=C(/C=C/C=C(/C=C/C2=C(CCCC2(C)
C)C)\C)\C)/C)/C
InChI: InChI=1S/C40H56/c1-31(19-13-21-33(3)25-27-37-35(5)23-15-29-39(37,7)8)17-11-12-18-32(2)20-14-22-34(4)26-28-38-36(6)24-16-30-40(38,9)10/h11-14,17-22,25-28H,15-16,23-24,29-30H2,1-10H3/b12-11+,19-13+,20-14+,27-25+,28-26+,31-17+,32-18+,33-21+,34-22+
InChIKey: OENHQHLEOONYIE-JLTXGRSLSA-N
beta-Carotene Uses
beta-Carotene (CAS NO.7235-40-7) is used for Vitamin A precursor. Ultraviolet screen.
beta-Carotene Toxicity Data With Reference
RTECS FI0329500
beta-Carotene Safety Profile
Hazard Codes of beta-Carotene (CAS NO.7235-40-7):  Xn
Xn
Risk Statements: 44-36/37/38-20/21/22
R44:Risk of explosion if heated under confinement.
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 7-15-18-36-26
S7:Keep container tightly closed.
S15:Keep away from heat.
S18:Handle and open container with care.
S36:Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 1
RTECS: FI0329500
F: 1-8-10-16
Hazardous Substances Data: 7235-40-7(Hazardous Substances Data)
beta-Carotene Specification
beta-Carotene , its cas register number is 7235-40-7. It also can be called (all-E)-1,1'-(3,7,12,16-Tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethylcyclohexene) ; Betacaroteno ; Betacarotenum ;
Carotaben ; KPMK ; Karotin ; Natural Yellow 26 ; Provatene ; Provitamin A ; Serlabo ; Solatene ; Solatene (caps) ; Zlut prirodni 26 ; all-trans-beta-Carotene ; beta-Carotene, all-trans- ; trans-B-Carotene . beta-Carotene (CAS NO.7235-40-7) is an organic compound and classified as a terpenoid. It is a strongly-coloured red-orange pigment abundant in plants and fruits.
Related Products
- beta-Carotene
- 7235-98-5
- 723-62-6
- 72363-26-9
- 7236-57-9
- 72366-48-4
- 723-69-3
- 72371-46-1
- 72372-66-8
- 7237-34-5
- 72373-81-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View