-
Name
n-Butyllithium
- EINECS 203-698-7
- CAS No. 109-72-8
- Article Data69
- CAS DataBase
- Density 0.68 g/mL at 20 °C
- Solubility vigorous reaction
- Melting Point -95 °C
- Formula C4H9Li
- Boiling Point 80 °C
- Molecular Weight 64.0565
- Flash Point 10 °F
- Transport Information UN 3399 4.3/PG 1
- Appearance clear yellow solution
- Safety 6-9-16-26-36/37/39-45-61-62-6A-46-43B-43-60-33-29-5
- Risk Codes 14/15-17-34-48/20-51/53-62-65-67-63-35-11-15-50/53-66
-
Molecular Structure
-
Hazard Symbols
 F,
F, C
C
- Synonyms 1-Butyllithium;Butyllithium;n-Butyllithium in Hexanes (15%soln & 23%soln);
- PSA 0.00000
- LogP 1.75410
Synthetic route
-
-
109-72-8
n-butyllithium

| Conditions | Yield |
|---|---|
| In toluene; pentane (Ar); 1.6 M n-BuLi in pentane was added at 0°C to soln. of ferrocene in toluene, stirred for 6 h at room temp.; filtered, washed with pentane, dried in vac., elem. anal.; | 93% |
-
-
109-72-8
n-butyllithium

-
-
384378-22-7
(CH3)2Ge(C5(CH3)4H)(C5H5)

| Conditions | Yield |
|---|---|
| In diethyl ether under N2, Schlenk technique; mixing at -78°C (BuLi in hexane), mixt. was warmed to room temp. and stirred for 15 h; solvent was removed, ppt. was washed with hexane, dried under vac.; elem. anal.; | 92% |
-
-
109-72-8
n-butyllithium

-
-
13292-87-0
dimethyl sulfide borane

-
B
-
82111-98-6
lithium [butyl(trihydrido)borate]

-
C
-
67335-72-2
LiHB(s-Bu)3

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane; dichloromethane under dry N2, hexane soln. of BuLi cooled to -20°C, H3B-SMe2 added to vigorously stirred soln. within 60 min, 2 hours later all volatiles removed in vac.; material suspended in hexane, THF added to form soln., solvents removed, oily material dissolved in CH2Cl2, pentane added to separate LiBH4, repetition of procedure gave slightly purer product; | A n/a B 91% C n/a |

-
-
109-72-8
n-butyllithium

-
-
134939-44-9
bis(tetramethylcyclopentadienyl)dimethylgermanium

| Conditions | Yield |
|---|---|
| In diethyl ether under N2, Schlenk technique; mixing at -78°C (BuLi in hexane), mixt. was warmed to room temp. and stirred for 15 h; solvent was removed, ppt. was washed with hexane, dried under vac.; elem. anal.; | 91% |
-
-
109-72-8
n-butyllithium

-
-
110-18-9
N,N,N,N,-tetramethylethylenediamine

-
-
1231953-83-5
trimethylamine borane

| Conditions | Yield |
|---|---|
| In hexane; toluene byproducts: (CH3)3N; under dry N2, hexane soln. of n-BuLi added to soln. of TMEDA in toluene, after 30 min added H3B-NMe3, heated for 1 h to reflux, soln. kept for 3 d at -25°C; elem. anal.; | A 10% B 90% |

-
-
109-72-8
n-butyllithium

-
-
478038-85-6
(CH3)2Ge(C5(CH3)4H)(C5H4CH3)

| Conditions | Yield |
|---|---|
| In diethyl ether under N2, Schlenk technique; mixing at -78°C (BuLi in hexane), mixt. was warmed to room temp. and stirred for 15 h; solvent was removed, ppt. was washed with hexane, dried under vac.; elem. anal.; | 90% |
-
-
7446-70-0
aluminium trichloride

-
-
109-72-8
n-butyllithium

-
-
443890-22-0
N-(2,6-bis(isopropyl)phenyl)-5-tert-butylpyrrolylaldimine

-
-
443890-21-9
[N-(2,6-bis(isopropyl)phenyl)-5-tert-butylpyrrolylaldiminato]aluminium(III) dichloride

| Conditions | Yield |
|---|---|
| In toluene under N2, Schlenk techques; n-BuLi was added to the soln. of ligand at -35°C, mixt. was warmed to room temp., stirred for 2 h, soln. was cooled to -35°C again and added to suspn. of AlCl3 with stirring,mixt. stirred at room temp. for 36 h; mixt. was filtered, washed with toluene, filtrate was concd., cooled to -35°C; elem. anal.; | 88% |

-
-
109-72-8
n-butyllithium

-
-
13292-87-0
dimethyl sulfide borane

-
B
-
82111-98-6
lithium [butyl(trihydrido)borate]

-
D
-
15243-31-9
Li{B(n-C4H9)4}

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane under dry N2, hexane soln. of n-BuLi cooled to -20°C, H3B-SMe2 added to vigorously stirred soln. within 4 h, resulting suspn. stirred overnight; filtered, detected by NMR; | A 13% B 87% C n/a D n/a |
| In hexane; dichloromethane; pentane under dry N2, hexane soln. of BuLi cooled to -20°C, H3B*SMe2 added to vigorously stirred soln. within 4 h, stirred overnight, solid filtered, solvent from filtrate removed; residue treated with 1:1 mixt. of CH2Cl2 and pentane, not isolated, detected in CH2Cl2-rich phase by NMR; |

-
-
109-72-8
n-butyllithium

-
-
519005-12-0, 439099-43-1
buta-1,3-dien-2-yltin trichloride

-
-
2244-38-4
buta-1,3-dien-2-yl-tributylstannane

| Conditions | Yield |
|---|---|
| In not given | 86% |
-
-
109-72-8
n-butyllithium

-
-
16390-61-7
1-phenyl-1,2-closo-C2B10H11

-
-
109-94-4
formic acid ethyl ester

-
-
16450-20-7
1-(CH(o-CB10H10CC6H5)OH)-2-(C6H5)-1.2-C2B10H10

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether; benzene nitrogen atmosphere; ether soln. of formate addn. at 50 to 60°C to benzene soln. of Li-salt of carborane (obtained from carborane and BuLi), stirring (1 h), treating with aq. HCl, extracting with ether; extract drying over MgSO4 and evapn., residue recrystn. (hexane); | 85% |

-
-
109-72-8
n-butyllithium

-
-
60-29-7
diethyl ether

-
-
443890-22-0
N-(2,6-bis(isopropyl)phenyl)-5-tert-butylpyrrolylaldimine

-
-
13499-05-3
hafnium tetrachloride

-
-
610270-20-7
[Hf(5-tert-butyl-2-[(2,6-diisopropylphenyl)aldimino]pyrrolyl)Cl2(μ-Cl)2Li(OEt2)2]

| Conditions | Yield |
|---|---|
| In diethyl ether under N2, Schlenk techques; n-BuLi was added to the soln. of ligand at -35°C, mixt. was warmed to room temp., stirred for 2 h, soln. was cooled to -35°C again and added to suspn. of HfCl4 with stirring,mixt. stirred at room temp. for 20 h; mixt. was filtered, washed with Et2O, ether filtrate was concd., cooled to -35°C; elem. anal.; | 85% |

-
-
109-72-8
n-butyllithium

-
-
4181-91-3
(R)-(-)-benzyl-α-d chloride

-
-
688-73-3
tri-n-butyl-tin hydride

-
-
108-18-9
diisopropylamine

-
-
84369-11-9
(S)-(-)-(.alfa.-deuteriobenzyl)tributyltin

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane to THF soln. of (i-Pr)2NH at 0°C added hexane soln. of BuLi, stirred at 0°C for 30 min, Bu3SnH added, stirred at 0°C for 30 min, soln. added to THF soln. of (R)-PhCHDCl at 0°C over 30 min, mixt. kept at 0°C for 5 h; added water, mixt. extd. with ether, sepd., washed with water and brine, extract dried with MgSO4, concd., chromd.; | 84% |

-
-
109-72-8
n-butyllithium

-
-
1231953-83-5
trimethylamine borane

-
B
-
82111-98-6
lithium [butyl(trihydrido)borate]

-
C
-
84280-42-2
lithium [(dimethylamino)methyl]trihydroborate

-
D
-
84280-43-3
lithium benzyltrihydroborate

| Conditions | Yield |
|---|---|
| In hexane; toluene under dry N2, refluxing for 30 min; | A 4% B 80% C 4% D 2% |
| In hexane; toluene under dry N2, refluxing for 2 d; | A 15% B 64% C 15% D 6% |

-
-
109-72-8
n-butyllithium

-
B
-
84280-32-0
lithium di-n-butylborohydride

-
C
-
67335-72-2
LiHB(s-Bu)3

-
D
-
15243-31-9
Li{B(n-C4H9)4}

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane under dry N2, soln. of BH3 in THF cooled to -78°C, hexane soln. of n-BuLi added with stirring, mixt. allowed to warm to -30°C within 1 h; not isolated, detected by NMR; | A 77% B 3% C 13% D 7% |

-
-
109-72-8
n-butyllithium

-
-
13292-87-0
dimethyl sulfide borane

-
B
-
82111-98-6
lithium [butyl(trihydrido)borate]

-
C
-
15243-31-9
Li{B(n-C4H9)4}

| Conditions | Yield |
|---|---|
| In hexane under dry N2, hexane soln. of n-BuLi cooled to -10°C, H3B-SMe2 added with stirring for few minutes, after 20 min all volatile material stripped in vac.; residue dissolved in THF, not isolated, detected by NMR; | A 18% B 77% C 5% |
| In hexane under dry N2, hexane soln. of n-BuLi added over period of 10 min to H3B-SMe2 with stirring; residue dissolved in minimum THF, not isolated, detected by NMR; | A 44% B 41% C 15% |


| Conditions | Yield |
|---|---|
| In hexane; toluene (N2 atmosphere); addn. of BuLi to susp. of C5H11PPh3Br (stirring, 0°C), addn. of soln. of Fe complex (after 5 min, -78°C), stirring (-78°C, 30 min, 0°C, 30 min), addn. of H2O (0°C),extraction (EtOAc), drying (MgSO4); evapn. (in vacuo), chromatography (SiO2 hexane/EtOAc); | 73% |

-
-
109-72-8
n-butyllithium

| Conditions | Yield |
|---|---|
| In hexane byproducts: LiCl, toluene; C4H9Li soln. added to suspn. of C20H8N4(BCl)2(C6H5CH3)4*(n)C6H5CH3 in hexane (-78 °C), warmed up to room temp., stirred (16 h); ppt. filtered, washed with hexane, extd. with CH2Cl2; detd. by MAS, UV, (1)H NMR, (11)B NMR; | 71% |

-
-
109-72-8
n-butyllithium

-
-
100-44-7
benzyl chloride

-
-
17534-15-5
1,2-benzenedithiole

-
-
116089-38-4
2-(Benzylthio)thiophenol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; carbon monoxide In tetrahydrofuran under N2; reaction of dithiol and BuLi at -78 °C; addn. of Fe-salt at room temp.; CO introduced for 2h; benzyl chloride added; stirring for 1h at room temp.; evapn.; excess of benzyl chloride removed by hexane; HCl and THF added; refluxed for 2h;; evapn.; treatment with H2O/CCl4; organic layer dried (Na2SO4), filtered and evapd.; distn. (180-220 °C, vacuum); elem. anal.;; | 70% |

-
-
109-72-8
n-butyllithium

-
-
53268-50-1
chloro(trifluoromethyl)disulfane

| Conditions | Yield |
|---|---|
| 68% | |
| 68% |
-
-
109-72-8
n-butyllithium

-
-
131498-79-8
1-trimethylsiloxy-3,7,10-trimethylgermatrane

-
-
1067-42-1
tetrabutylgermanium

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: LiOSi(CH3)3; a suspn. of germatrane in THF was treated with an excess of n-BuLi in n-hexane at room temp. for 4 h; evap., filtered; | 65% |
-
-
1271-86-9
N,N-dimethylaminomethylferrocene

-
-
109-72-8
n-butyllithium

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
162762-18-7, 212901-69-4, 174225-69-5
2-(N,N-dimethylaminomethyl)ferrocenecarboxaldehyde

| Conditions | Yield |
|---|---|
| In not given addn. of LiBu (1 equiv., room temp.), stirring (24 h), addn. of DMF (-78°C), stirring (4 h, room temp.), hydrolysis; extn. (Et2O), chromy. (alumina, Et2O/pentane 1:2); elem. anal.; | 62% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran a suspn. of germatrane in THF was treated with an excess of n-BuLi in n-hexane at room temp. for 4 h; | 61% |

| Conditions | Yield |
|---|---|
| In hexane under dry N2, refluxing for 20 h; | 60% |
-
-
26743-81-7
tetracarbonyl-bis(diphenylphosphino)methane-molybdenum(0)

-
-
109-72-8
n-butyllithium

-
-
115-09-3
methylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of Mo-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

-
-
16743-46-7
tetracarbonyl-bis(diphenylphosphino)methane-chromium(0)

-
-
109-72-8
n-butyllithium

-
-
115-09-3
methylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of Cr-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

-
-
109-72-8
n-butyllithium

-
-
41830-14-2
tetracarbonyl-bis(diphenylphosphino)methane-tungsten(0)

-
-
115-09-3
methylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of W-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

-
-
26743-81-7
tetracarbonyl-bis(diphenylphosphino)methane-molybdenum(0)

-
-
109-72-8
n-butyllithium

-
-
107-27-7
ethylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of Mo-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

-
-
16743-46-7
tetracarbonyl-bis(diphenylphosphino)methane-chromium(0)

-
-
109-72-8
n-butyllithium

-
-
107-27-7
ethylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of Cr-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

-
-
109-72-8
n-butyllithium

-
-
41830-14-2
tetracarbonyl-bis(diphenylphosphino)methane-tungsten(0)

-
-
107-27-7
ethylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of W-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

-
-
26743-81-7
tetracarbonyl-bis(diphenylphosphino)methane-molybdenum(0)

-
-
109-72-8
n-butyllithium

-
-
100-56-1
phenylmercury(II) chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of LiBu (hexane soln.) to soln. of Mo-complex (0°C), stirring (2 h), addn. of Hg-compd., warming (room temp.), stirring (2 h); THF removal (reduced pressure), recrystn. (CH2Cl2-methanol); | 60% |

n-Butyllithium Chemical Properties
Empirical Formula: C4H9Li
Molecular Weight: 64.0553
Flashing point: 10 °F
Density: 0.68 g/mL at 20 °C
Boiling Point: 80 °C
Melting point: -95 °C
Storage tempreture: 2-8 °C
Water solubility: Vigorous reaction
Sensitive: Air & moisture sensitive
Appearance: Clear yellow solution
Structure of n-Butyllithium (CAS NO.109-72-8):
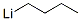
IUPAC Name: Lithium butane
Canonical SMILES: [Li+].CCC[CH2-]
InChI: InChI=1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;/q-1;+1
InChIKey: DLEDOFVPSDKWEF-UHFFFAOYSA-N
Product Category of n-Butyllithium (CAS NO.109-72-8): Organometallics;Alkyl Metals;Grignard Reagents & Alkyl Metals;Li (Lithium) Compounds;Synthetic Organic Chemistry;Typical Metal Compounds
n-Butyllithium Uses
n-Butyllithium (CAS NO.109-72-8) is principally valued as a catalyst for the industrial polymerization of dienes, such as butadiene: C4H9Li + CH2=CH-CH=CH2 → C4H9-CH2-CH=CH-CH2Li .
Isoprene can be polymerized stereospecifically in this way. Also of commercially importance is the use of butyllithium for the production of styrene-butadiene polymers. Even ethylene will insert into BuLi.
n-Butyllithium Production
The standard preparation for n-BuLi is reaction of bromobutane or chlorobutane with Li metal:
2 Li + C4H9X → C4H9Li + LiX
where X = Cl, Br
n-Butyllithium Safety Profile
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection. S36/37/39:Wear suitable protective clothing, gloves and eye/face protection. Probably very toxic. Solutions of greater than 20% will ignite spontaneously in air. Ignites on contact with water or CO2. May cause potentially explosive polymerization of styrene. Extremely flammable. To fight fire, use dry chemical; see special instructions of manufacturer. See also LITHIUM COMPOUNDS and BUTYLLITHIUM.
Hazard Codes:  F,
F, C,
C, N
N
Risk Statements: 14/15-17-34-48/20-51/53-62-65-67-63-35-11-15-50/53-66
R11:Highly flammable.
R14 :Reacts violently with water.
R15:Contact with water liberates extremely flammable gases.
R17:Spontaneously flammable in air.
R20:Harmful by inhalation.
R35:Causes severe burns.
R48:Danger of serious damage to health by prolonged exposure.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R62:Risk of impaired fertility.
R65:Harmful: may cause lung damage if swallowed.
R67:Vapours may cause drowsiness and dizziness.
R66:Repeated exposure may cause skin dryness or cracking.
Safety Statements: 6-9-16-26-36/37/39-45-61-62-6A-46-43B-43-60-33-29-5
S9:Keep container in a well-ventilated place.
S16:Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S33:Take precautionary measures against static discharges.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S43:In case of fire use ... (there follows the type of fire-fighting equipment to be used.)
S46:If swallowed, seek medical advice immediately and show this container or label.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S62:If swallowed, do not induce vomitting; seek medical advice immediately and show this container or label.
n-Butyllithium Specification
n-Butyllithium , its cas register number is 109-72-8. It also can be called Lithium, butyl- ; and Butyllithium . It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: Do not induce vomiting. Get medical aid immediately. Notes to physician: Treat supportively and symptomatically.
In addition, n-Butyllithium (CAS NO.109-72-8) is air sensitive, moisture sensitive, and light sensitive, and reacts violently with water. It contacts with water liberates highly flammable gases., acids, alcohols, carbon dioxide, oxygen, hydrogen, organic halides, and you must not take it with incompatible materials, ignition sources, exposure to air, exposure to moist air or water, direct sunlight.. And also prevent it to broken down into hazardous decomposition products: Carbon monoxide, carbon dioxide, butane, lithium oxide.
Related Products
- n-Butyllithium
- 1097-35-4
- 109737-05-5
- 109-73-9
- 109-74-0
- 109741-38-0
- 109744-49-2
- 109745-15-5
- 109746-09-0
- 109750-35-8
- 109-75-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View