Dayang Chem (Hangzhou) Co.,Ltd.
DayangChem exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in China, DayangChem is your best choice. Pls contact with us freely for getting detailed product spe
Cas:1310-65-2
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquiryHangzhou Dingyan Chem Co., Ltd
Test Items Standard Test Results Appearance White tiny crystalline White tiny crystalline Assay
Cas:1310-65-2
Min.Order:1 Gram
FOB Price: $100.0 / 500.0
Type:Manufacturers
inquiryAlity Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Hebei yanxi chemical co.,LTD.
Hebei yanxi chemical co., LTD is a professional research, development and production of lead acetate benzene acetamide enterprise backbone members by local well-known entrepreneurs and professional senior engineers in the party's "low carb
Cas:1310-65-2
Min.Order:1 Metric Ton
FOB Price: $1.0 / 3.0
Type:Trading Company
inquiryHenan Tianfu Chemical Co., Ltd.
Product Name: Lithium hydroxide Synonyms: Lithium Hydroxide Anhydrous [for General Organic Chemistry];"Lithium hydroxide, anhydrous/ 99%";Lithium hydroxide ,99% [anhydrous];LithiuM hydroxide, anhydrous, pure, 98% 500GR;LITHIU
Cas:1310-65-2
Min.Order:1 Gram
FOB Price: $8900.0
Type:Lab/Research institutions
inquiryHenan Sinotech Import&Export Corporation
CAS No.: 1310-65-2 MW: 23.95 MF: LiOH MELTING POINT:462 °C BOLING POINT: 925°C PH: 12-14 (50g/l, H2O, 50℃) Properties: White crystals;Soluble in water and slightly soluble in ethanol Water solubility(100
Cas:1310-65-2
Min.Order:5 Metric Ton
FOB Price: $1.0 / 2.0
Type:Other
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:1310-65-2
Min.Order:1 Kilogram
FOB Price: $24.0 / 34.0
Type:Trading Company
inquiryHebei Mojin Biotechnology Co.,Ltd
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in lead acetate, diphenyl ethylamine and other chemical raw materials and chemical reagents research and development production enterprises. Our business covers more than 30 countrie
Cas:1310-65-2
Min.Order:1 Kilogram
FOB Price: $80.0 / 100.0
Type:Trading Company
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:1310-65-2
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
Zibo Hangyu Biotechnology Development Co., Ltd
Zibo Hangyu Biotechnology Development Co., Ltd is a leading manufacturer and supplier of chemicals in China. We develop produce and distribute high quality pharmaceuticals, intermediates, special chemicals and OLED intermediates and other fine chemi
Cas:1310-65-2
Min.Order:10 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryHANGZHOU YUNUO CHEMICAL CO.,LTD
Superior quality, moderate price & quick delivery. Appearance:white crystal powder Storage:Stored in cool, dry and ventilation place; Away from fire and heat Package:25kg/drum, or as per your request. Application:Used as Pharmaceutical Intermed
Zibo Dorne chemical technology co. LTD
Product Details Grade: pharmaceutical grade Purity:99%+ ProductionCapacity: 1000 Kilogram/Month Scope of use: For scientific research only(The product must be used legally) Our Advantage 1. Best quality with competitive price. 2. Quick shipping,
Cas:1310-65-2
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHenan Wentao Chemical Product Co., Ltd.
We are leading fine chemicals supplier in China with ISO certificate, Our main business covers the fields below: 1.Noble Metal Catalysts (Pt.Pd...) 2.Organic Phosphine Ligands (Tert-butyl-phosphine.Cyclohexyl-phosphine...) 3.OLED
Cas:1310-65-2
Min.Order:1 Gram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryHangzhou Ocean Chemical Co., Ltd.
Ocean inorganic department is a professional supplier and exporter engaging in inorganic chemical materials and metal organic compounds. Over the past years, our company is committed to improving the product quality and developing new products, in
Cas:1310-65-2
Min.Order:1 Kilogram
FOB Price: $1.0
Type:Lab/Research institutions
inquiryGIHI CHEMICALS CO.,LIMITED
high purity,in stock Package:25kg/drum,or as per customers'demand Application:API,or Intermediates,fine chemicals Transportation:air,sea,courier
Aecochem Corp.
Our clients, like BASF,CHEMO,Brenntag,ASR,Evonik,Merck and etc.Appearance:COA Storage:in stock Application:MSDS/TDS
Zhuozhou Wenxi import and Export Co., Ltd
Product Description Description & Specification Category Pharmaceutical Raw Materials, Fine Chemicals, Bulk drug Standard Medical standard
Cas:1310-65-2
Min.Order:1 Kilogram
FOB Price: $112.0
Type:Trading Company
inquiryAntimex Chemical Limied
Ansciep Chemical is a professional enterprise manufacturing and distributing fine chemicals and speciality chemicals. We have been dedicated to heterocycle compounds and phenyl rings for tens of years. This is our mature product for export. Our quali
Xiamen AmoyChem Co.,Ltd
Amoychem is committed to providing the top-quality chemical products and services Internationally. We offer our customers with friendly, professional service and reliable, high performance products that have been manufactured according to the accredi
Hubei Langyou International Trading Co., Ltd
2018 New hot sale product CAS 1310-65-2 Lithium Hydroxide Anhydrous Application:2018 New hot sale product CAS 1310-65-2 Lithium Hydroxide Anhydrous
Cas:1310-65-2
Min.Order:0
Negotiable
Type:Other
inquiryWuhan ZeShanCheng Biomedical Technology Co., Ltd.
1,we produce and sell good chemicals around the world. 2,our success rate is about 95%. this means, if customer order is accepted, the probability that the customer will obtain the ordered substances, is 95%. 3,our staff consists of highl
Hangzhou ZeErRui Chemical Co., Ltd.
Hangzhou ZeErRui Chemical Co., Ltd. located in Lingang industrial areas, our plant covers an area of 6000 square meters.ZeErRui dedicated to the development, production and marketing of chemicals. We have earned ourselves a good reputation at home an
Cas:1310-65-2
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryHunan Russell Chemicals Technology Co.,Ltd
low price and high purityAppearance:solid or liquid Storage:in sealed air resistant place Package:As customer require Application:Pharma;Industry;Agricultural Transportation:by sea or by airplane Port:any port in China
Cas:1310-65-2
Min.Order:0
Negotiable
Type:Trading Company
inquiryHunan Longxianng Runhui Trading Co.,Ltd
2018 New hot sale product CAS 1310-65-2 Lithium Hydroxide AnhydrousAppearance:powder Storage:keep dry Package:25kg/bag or follow your requirement Application:pharmaceutical intermediates Transportation:By express (Door to door) such as FEDEX, DHL, EM
Cas:1310-65-2
Min.Order:0
Negotiable
Type:Lab/Research institutions
inquiryBeantown Chemical
in stock Application:1310-65-2
Cas:1310-65-2
Min.Order:0
Negotiable
Type:Trading Company
inquiryYurui(Shanghai)Chemical Co.,Ltd
How May We Serve You We can Supply HPLC\HMMR\MS report for you ,pls feel free to contact us! Capability on chemical synthesis ● Shanghai High-Tech Enterprises ● Strong R&D Team from USA, Japan, Korea ● 65000M2+ Factory, 300+ reac
Cas:1310-65-2
Min.Order:100 Gram
Negotiable
Type:Lab/Research institutions
inquiryHebei Yingong New Material Technology Co.,Ltd .
Our advantages: As an experienced company, from the production to quality testing, we strictly check every step to ensure that the quality of each product can meet the standard. We also offer specialized logistic service including export declaration
Cas:1310-65-2
Min.Order:1 Kilogram
FOB Price: $110.0 / 130.0
Type:Trading Company
inquiryHENAN SUNLAKE ENTERPRISE CORPORATION
Lithium hydroxide Basic information Product Name: Lithium hydroxide Synonyms: LiOH;Lithium hydroxide (Li(OH));lithiumhydroxide(li(oh));lithiumhydroxideanhydrous;Lit
Changzhou Extraordinary Pharmatech co.,LTD
Changzhou Extraordinary Pharmatech co., LTD. As a leading chemical manufacturer and supplier in China.DAS authentication is passed.We can provide the popular precursor chemicals, we have our own strong R & D team, have our own laboratories and fa
Synthetic route
-
-
177723-35-2, 64332-99-6
[CeCl3(H2O)]

-
-
917-54-4
methyllithium

-
A
-
34557-54-5
methane

-
B
-
7790-86-5
cerium chloride

-
C
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (vac.); | A 40% B n/a C n/a |


| Conditions | Yield |
|---|---|
| In gaseous matrix Kinetics; byproducts: H; react. of Li atoms and water vapour (excess), N2 bath gas, T = 850, 900, 925, 950, 973, 1000K; monitoring by time-resolved laser induced fluorescence (670.7 nm); | |
| In neat (no solvent) on moist air, fast reaction;; | |
| In tetrahydrofuran reactn. of excess of lithium with H2*O; (17)O/(18)O-enrichment: about 10% (17)O, about 40% (18)O; |

| Conditions | Yield |
|---|---|
| In water formation of a lot of Li2O and a few LiOH;; determination with electron diffraction;; |

-
-
7732-18-5
water

-
-
7789-20-0
water-d2

-
-
7439-93-2
lithium

-
A
-
12159-20-5
LiOD

-
B
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water dissolving Li in mixt. of H2O and (2)H2O under Ar atmosphere; H/(2)H ratio in electrolyte 0.22+/-0.01; |


| Conditions | Yield |
|---|---|
| byproducts: NH3; |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane (N2); using Schlenk techniques; addn. of soln. of n-BuLi in hexane to cooled soln. of water in THF; |

| Conditions | Yield |
|---|---|
| In not given byproducts: HD; formation from LiD and water besides HD;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; analysis of the equilibrium of the reaction, determination of partial pressure of CO2 depending on temp.;; | |
| In neat (no solvent) Kinetics; analysis of the equilibrium of the reaction, determination of partial pressure of CO2 depending on temp.;; |


| Conditions | Yield |
|---|---|
| Li2O is heated at 673 K in Ar saturated atmosphere with water vapour (parial pressure 1.5 kPa).; | |
| In water vaporization of aq. Li2O soln., strong heating of residue;; | |
| In neat (no solvent) formation with Li2O and the moisture of the air;; | |
| In water vaporization of aq. Li2O soln., strong heating of residue;; | |
| In neat (no solvent) formation with Li2O and the moisture of the air;; |

| Conditions | Yield |
|---|---|
| With barium sulfate In water pptn. from Li2SO4 soln. with baryte, filtration, vaporization;; melting in platinum crucible;; | |
| With barium sulfate In water pptn. from Li2SO4 soln. with baryte, boiling very diluted soln. in platinum or silver crucible, melting at 445°C;; pouring on silver plate, crushing; LiOH contains water;; | |
| With baryte In water pptn. from Li2SO4 soln. with baryte, boiling very diluted soln. in platinum or silver crucible, melting at 445°C;; pouring on silver plate, crushing; LiOH contains water;; | |
| With baryte In water pptn. from Li2SO4 soln. with baryte, filtration, vaporization;; melting in platinum crucible;; |

| Conditions | Yield |
|---|---|
| With water In melt hydrolytic decompn.; | |
| With H2O In melt hydrolytic decompn.; |

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| With air In neat (no solvent) formation by reaction of LiCl with dry air;; | |
| With air; H2O In melt byproducts: Li2CO3, HCl; decompn. on melting on air;; | |
| With air |

| Conditions | Yield |
|---|---|
| With water In water equilibrium; depending on temp. (25.0-99.5°C); | |
| With H2O In water |


| Conditions | Yield |
|---|---|
| With air In neat (no solvent) formation on reaction with air at normal temp.;; identification by electron diffraction;; | |
| With air In neat (no solvent) formation on reaction with air at normal temp.;; identification by electron diffraction;; |

-
-
7439-93-2
lithium

-
A
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
B
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| With hydrogen In neat (no solvent) in the presence of H2, formation of LiOH and Li2O;; | |
| With H2 In neat (no solvent) in the presence of H2, formation of LiOH and Li2O;; |


-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| With lime milk | |
| With calcium hydroxide In not given on heating spodumene to 1100-1150°C and then treating with lime milk at 100-205°C under pressure;; |

| Conditions | Yield |
|---|---|
| In not given |

| Conditions | Yield |
|---|---|
| In not given byproducts: NaF, Si(OH)4; reaction of Li2SiF6 with NaOH, formation of LiOH, NaF and Si(OH)4;; |

| Conditions | Yield |
|---|---|
| In not given byproducts: Na2SO4; on cooling reaction of Li2SO4 with NaOH until Na2SO4 is crystallized, then crystallizing of pure LiOH;; |

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| With calcium oxide In not given byproducts: Ca(OH)2, CaO*Al2O3; mixture is cooled with air and crushed wet, soluble LiOH is formed;; |

| Conditions | Yield |
|---|---|
| With Mg-amalgam In water byproducts: Mg(OH)2, NH2OH; exotherm reaction; drying over H2SO4; | |
| With Mg-amalgam In water byproducts: Mg(OH)2, NH2OH; exotherm reaction; drying over H2SO4; |

| Conditions | Yield |
|---|---|
| In water byproducts: CO2; equil.;; | |
| In water determination of equil. in boiling soln.;; | |
| In water determination of equil. in boiling soln.;; | |
| In water byproducts: CO2; equil.;; |

| Conditions | Yield |
|---|---|
| In not given reaction of Li2SiO3 with Ca(OH)2;; | |
| In water 5-6 h boiling of mixt. of Li2CO3, Ca(OH)2 and H2O with stirring, filtration, vaporization of filtrate;; melting in silver crucible;; | |
| In water 5-6 h boiling of mixt. of Li2CO3, Ca(OH)2 and H2O with stirring, filtration, vaporization of filtrate;; melting in silver crucible;; | |
| In not given reaction of Li2SiO3 with Ca(OH)2;; |

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| With methyllithium In water aq. soln. of Li2SO4 reacts with lime to LiOH;; | |
| With lime In water |

| Conditions | Yield |
|---|---|
| In water mixing hot satd. soln. of Ba(OH)2 with small excess of hot satd. Li2SO4 soln., decantation of clear soln. after storage for few days;; carbonate free aq. soln. of LiOH;; | |
| In not given formation of pure LiOH;; | |
| In water mixing hot satd. soln. of Ba(OH)2 with small excess of hot satd. Li2SO4 soln., decantation of clear soln. after storage for few days;; carbonate free aq. soln. of LiOH;; |

| Conditions | Yield |
|---|---|
| Kinetics; other Radiation; Irradiation with a CW CO2 laser at an output power of P=25 W.; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2O; heating in stream of dry H2 at various temp.;; | |
| below 140°C in dry H2 stream; | |
| byproducts: H2O; 140°C; X-ray diffraction, atomic absorption; |

-
C
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water byproducts: H2O; hydrolysis, in nickel autoclave, above 200°C; |


| Conditions | Yield |
|---|---|
| With water In water with water and exclusion of air, decomposition in PH3, LiOH and NH3; sinilary reaction with dild. acids;; | |
| With H2O In water with water and exclusion of air, decomposition in PH3, LiOH and NH3; sinilary reaction with dild. acids;; |


| Conditions | Yield |
|---|---|
| In water with water decomposition in PH3 and LiOH;; | |
| In water with water decomposition in PH3 and LiOH;; |
-
-
1143534-34-2
methyl 3-(4-(tert-butoxycarbonylamino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamido)-3-(4-chlorophenyl)propanoate

-
-
1310-65-2
lithium hydroxide

-
-
1143535-47-0
methyl 3-(4-(tert-butoxycarbonylamino)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamido)-3-(4-chlorophenyl)propanoate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In ethylene glycol at 215℃; for 36h; Time; Solvent; Autoclave; High pressure; | 100% |


-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water (Et3NH)2(B12H12) was added to 2 equiv. aq. soln. LiOH and heated until soln. became clear; soln. was evapd. to dryness and dried in vacuo at 150-200°C; | 99% |
| In water byproducts: Et3N, H2O; purging of soln. with N2 (80°C, 2 h); filtration., evapn. of soln. (vac., 80°C), drying (vac. 140°C); | 91% |

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water neutralized; | 99% |

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol by dissolving mixt. of educts (in 1:3 molar ratio) in small amt. of H2O on steam bath; addn. of i-propanol to hot soln., cooling to room temp.; filtering under N2; washing with i-propanol and anhydrous ether; purified by dissolving in ether and pptn. with i-propanol; | 98% |
| In water; isopropyl alcohol |
-
-
142453-07-4
tris(2,6-dimethoxyphenyl)borane

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In methanol; water | 98% |

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In methanol; water for 3h; Reflux; | 98% |
-
-
52102-17-7
9-ethyl-9-borabicyclo[3.3.1]nonane

-
-
1310-65-2
lithium hydroxide

-
-
137669-89-7
lithium 1,5-cyclooctanediylethylhydroxoborate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran Ar atmosphere; addn. of B-compd. in THF to LiOH in THF (25 min), stirring (room temp.); partial evapn. (12 Torr), drying (20°C, 0.001 Torr), not pure, elem. anal.; | 97% |
-
-
926304-10-1
piperazinium oxalate monohydrate

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water High Pressure; Ni(OH)2, (C4H12N2)(C2O4)*H2O, LiOH and H2O heated in autoclave to 180°C for 72 h, cooled; elem. anal.; | 97% |


-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In methanol; water 20°C; 48 h;; elem. anal.;; | 96% |

| Conditions | Yield |
|---|---|
| In water H2O, an aq. soln. of H2O2, LiOH soln., and U salt combined and stirred; MeOH or EtOH or i-PrOH added rapidly, filtered; | 96% |

-
-
2402-77-9
2,3-dichloro-pyridine

-
-
444993-18-4
2-(prop-2-yloxycarbonyl)-5-trfluoromethylphenylboronic acid

-
-
1310-65-2
lithium hydroxide

-
-
1423022-47-2
2-(3-chloro-pyridin-2-yl)-4-trifluoromethyl-benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichloro-pyridine; 2-(prop-2-yloxycarbonyl)-5-trfluoromethylphenylboronic acid With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In 1,4-dioxane; water at 30 - 110℃; for 20h; Inert atmosphere; Stage #2: lithium hydroxide In 1,4-dioxane; water at 70℃; | 96% |


-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In ethanol; water in H2O/EtOH at room temp.; | 95% |

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In ethanol; water in H2O/EtOH at room temp.; | 95% |
-
-
1370656-27-1
(S)-methyl 1-((6-(hydroxymethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxylate

-
-
1310-65-2
lithium hydroxide

-
-
1370656-24-8
lithium (S)-1-((6-(hydroxymethyl)pyridin-2-yl)methyl)pyrrolidine-2-carboxylate

| Conditions | Yield |
|---|---|
| In methanol; water for 3h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| In water for 18h; Schlenk technique; Inert atmosphere; | 95% |


-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In ethanol at 70℃; | 95% |

| Conditions | Yield |
|---|---|
| In water pH=7; | 95% |

| Conditions | Yield |
|---|---|
| In water pH 7-7.5; 2 h boiling on water bath; filtering, drying; | 94% |
| In water pH 7-7.5; 2 h boiling on water bath; filtering, drying; | 94% |
| In neat (no solvent, solid phase) for 5 mo; not isolated, detected by XRD; | |
| Heating; |

| Conditions | Yield |
|---|---|
| With ethanol In ethanol byproducts: CH3CHO, H2O; High Pressure; equimolar amount of LiOH and V2O5 put into autoclave, filled with ethanol up to 80% of total volume, kept at 160°C under autogeneous pressure for 24 h, cooled to room temp. naturally; precipitate filtered, washed with ethanol, dried at 100°C for 2 h; | 94% |
-
-
7732-18-5
water

-
-
7550-45-0
titanium tetrachloride

-
-
5949-29-1
citric acid monohydrate

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water TiCl4 added dropwise to a water on ice bath, acid added at room temp., pH adjusted to 2 (LiOH); stored for several d at room temp.; elem. anal.; | 93.7% |


| Conditions | Yield |
|---|---|
| With citric acid In tetrahydrofuran; water Fe compd. and LiOH (1:2.5 molar ratio) stirred in THF/H2O (30/1, v/v) atroom temp. for 48 h; soln. poured in 10% citric acid, extd. (ethyl acetate), washed (H2O), dried (Na2SO4), filtered, evapd. (vac.), elem. anal.; | 93% |
-
-
1310-65-2
lithium hydroxide

-
-
1025458-87-0
[HO(CH2)10C(C4HN(CH3)2)2BF2]

| Conditions | Yield |
|---|---|
| With potassium acetate In not given | 92% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 91.2% |

| Conditions | Yield |
|---|---|
| Stage #1: C35H42N4O9; lithium hydroxide In tetrahydrofuran; methanol; water at 0 - 20℃; for 12h; Stage #2: With trifluoroacetic acid In tetrahydrofuran; methanol; water at 0 - 20℃; for 5h; | 91% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol at 0 - 28℃; for 48h; Inert atmosphere; Schlenk technique; | 91% |
-
-
117319-08-1
P-trans-Cl{Rh(o-diphenylphosphinophenylamine)(CO)Cl}

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water; acetone LiOH in water added to Rh-complex in acetone under N2-atmosphere; stirred for 2 h;; water added with stirring, ppt. filtered off and washed with water, elem. anal;; | 90% |
-
-
99727-69-2
[Pt(NHC(C6H5)N(C4H9)CH2CH2NH(C4H9))Cl(NC(C6H5))](1+)*Cl(1-)

-
-
1310-65-2
lithium hydroxide

-
-
99727-71-6
[Pt(NHC(C6H5)N(C4H9)CH2CH2NH(C4H9))Cl(NHC(O)C6H5)]*CH3OH

| Conditions | Yield |
|---|---|
| With methanol In methanol to soln.of Pt complex in MeOH is added dropwise soln. of stoich. amt. of LiOH in MeOH; evapd. in vac., extd. with CH2Cl2, evapd., recrystd. from MeOH; elem. anal.; | 90% |
-
-
37366-09-9
dichloro(benzene)ruthenium(II) dimer

-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| In water-d2 5 equiv. of LiOH added to soln. of (C6H6RuCl2)2 and C4H10N2(CH2C5H2N(OH)2)2; not isolated, detected by NMR; | 90% |


-
-
1310-65-2
lithium hydroxide

| Conditions | Yield |
|---|---|
| With citric acid In tetrahydrofuran; water Fe compd. and LiOH (1:2.5 molar ratio) stirred in THF/H2O (30/1, v/v) atroom temp. for 48 h; soln. poured in 10% citric acid, extd. (ethyl acetate), washed (H2O), dried (Na2SO4), filtered, evapd. (vac.), elem. anal.; | 90% |
Related products
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View




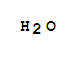









 C
C