-
Name
3,5-Dichloropyridine
- EINECS 219-537-9
- CAS No. 2457-47-8
- Article Data22
- CAS DataBase
- Density 1.388 g/cm3
- Solubility
- Melting Point 65-67 °C
- Formula C5H3Cl2N
- Boiling Point 173.7 °C at 760 mmHg
- Molecular Weight 147.992
- Flash Point 73.4 °C
- Transport Information
- Appearance White to yellow powder
- Safety 26-39-36
- Risk Codes 22-37/38-41-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms NSC 60546;
- PSA 12.89000
- LogP 2.38840
Synthetic route
-
-
15177-57-8
3,5-dicholoropyridine N-oxide

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| With 2,3-dimethyl-2,3-butane diol; bis(N,N-dimethylformamide)dichloridodioxidomolybdenum(VI) In N,N-dimethyl acetamide at 130℃; Microwave irradiation; Sealed tube; Green chemistry; chemoselective reaction; | 95% |
-
-
16063-70-0
2,3,5-trichloropyridine

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In water at 65 - 95℃; for 1 - 5.25h; Product distribution / selectivity; | 87.3% |
-
-
99749-00-5
lithioacetonitrile

-
-
153463-65-1
3,5-dichloropyridine-4-carbonitrile

-
A
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.0833333h; | A n/a B 73% |


| Conditions | Yield |
|---|---|
| With sodium acetate; acetic acid; zinc at 100℃; for 5.5h; Product distribution / selectivity; | 65% |
| With acetic acid; zinc In water at 81 - 82℃; for 30h; Product distribution / selectivity; | 44.6 %Chromat. |
| With acetic acid; zinc In 1,4-dioxane; water at 81 - 82℃; for 29h; Product distribution / selectivity; | 60.8 %Chromat. |
-
-
221615-75-4
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone

-
A
-
2457-47-8
3,5-dichloropyridine

-
B
-
202409-33-4
etoricoxib

| Conditions | Yield |
|---|---|
| With ammonium acetate In propionic acid at 125℃; | A n/a B 62% C 15% |

-
-
917-54-4
methyllithium

-
-
153463-65-1
3,5-dichloropyridine-4-carbonitrile

-
A
-
100868-46-0
3,5-dichloro-4-picoline

-
B
-
2457-47-8
3,5-dichloropyridine

-
D
-
402561-62-0
methyl 3,5-dichloro-4-pyridyl imine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -75℃; for 0.333333h; | A 12% B 12% C 9% D 57% |

-
-
917-54-4
methyllithium

-
-
153463-65-1
3,5-dichloropyridine-4-carbonitrile

-
A
-
100868-46-0
3,5-dichloro-4-picoline

-
B
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.333333h; | A 3% B 45% C 32% |

-
-
16063-70-0
2,3,5-trichloropyridine

-
A
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In dimethyl sulfoxide Heating; | A 18% B 28% |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride |
-
-
4454-05-1
2-methoxy-3,4-dihydro-2H-pyran

-
A
-
626-60-8
3-Chloropyridine

-
B
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| With chlorine Eintragen des Reaktionsgemisches in eine heisse wss. Loesung von Ammoniumchlorid und Eisen(III)-chlorid; | |
| With chlorine Eintragen des Reaktionsgemisches in eine heisse wss. Loesung von Ammoniumchlorid und Eisen(III)-chlorid; |
-
-
22353-34-0
5-chloropyridin-3-amine

-
-
2457-47-8
3,5-dichloropyridine
-
-
81719-53-1
3,5-dichloropyridine-2-carboxylic acid

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| Erhitzen auf Temperaturen oberhalb des Schmelzpunkts; |
-
-
13958-93-5
3,5-dichloropyridine-4-carboxylic acid

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| at 230℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With chlorine at 115 - 120℃; |

| Conditions | Yield |
|---|---|
| With chlorine at 170℃; | |
| With chlorine at 200℃; |
-
-
24903-95-5
nopinone

-
A
-
17180-94-8
5-chloropyrimidine

-
B
-
2457-47-8
3,5-dichloropyridine

-
C
-
117648-74-5
5-Chloro-10,10-dimethyl-3-aza-tricyclo[7.1.1.02,7]undeca-2(7),3,5-triene

| Conditions | Yield |
|---|---|
| Multistep reaction. Yields of byproduct given; | |
| Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| With 4-bromoanisole cation radical In acetonitrile at 23℃; Rate constant; Mechanism; |
-
-
23029-86-9
N-methyl-3,5-dichloropyridinium iodide

-
-
603-35-0
triphenylphosphine

-
A
-
2457-47-8
3,5-dichloropyridine

-
B
-
2065-66-9
methyl-triphenylphosphonium iodide

| Conditions | Yield |
|---|---|
| In acetonitrile at 115℃; Rate constant; Thermodynamic data; k2=15.1s-1M-1; var. temp. ΔS*=9.1 e.u. ΔH*=29.7kcal/mol; |

-
-
23029-86-9
N-methyl-3,5-dichloropyridinium iodide

-
-
74-88-4
methyl iodide

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; Kinetics; Equilibrium constant; Thermodynamic data; ΔG(excit.), ΔH(excit.), ΔS(excit.); |

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| With glycerol beim Destillieren; |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride at 200℃; |
-
-
110-86-1
pyridine

-
-
7782-50-5
chlorine

-
A
-
33216-52-3
3,4,5-trichloropyridine

-
B
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| at 220℃; |

-
-
110-86-1
pyridine

-
-
7782-50-5
chlorine

-
A
-
626-60-8
3-Chloropyridine

-
B
-
33216-52-3
3,4,5-trichloropyridine

-
C
-
2176-62-7
2,3,4,5,6-pentachloropyridine

-
D
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| at 170℃; beim Behandeln des Pyridin-hydrochlorids; |

-
-
5439-04-3
3,4,5-trichloropicolinic acid

-
-
10034-85-2
hydrogen iodide

-
A
-
81719-53-1
3,5-dichloropyridine-2-carboxylic acid

-
B
-
2457-47-8
3,5-dichloropyridine

-
-
136590-83-5
3,5-dichloropyridine-4-carboxaldehyde

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine-4-carboxaldehyde With phenyllithium In diethyl ether; hexane at -75℃; for 2h; Stage #2: With hydroxide In water |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: thionyl chloride / 180 - 220 °C / im Rohr und Zersetzen des Reaktionsprodukts mit Wasser 2: 230 °C / im Rohr View Scheme |
-
-
846045-08-7, 858443-51-3
3,5-dichloro-4-hydroxy-pyridine-2-carboxylic acid

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: POCl3; PCl5 3: ethanolic alkaline solution 4: aqueous HI; red phosphorus 5: Erhitzen auf Temperaturen oberhalb des Schmelzpunkts View Scheme |

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 2: ethanolic alkaline solution 3: aqueous HI; red phosphorus 4: Erhitzen auf Temperaturen oberhalb des Schmelzpunkts View Scheme |

| Conditions | Yield |
|---|---|
| at 100℃; under 4500360 Torr; for 72h; | 100% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
107-18-6
allyl alcohol

-
-
897396-03-1
3-chloro-5-propen-cis-yloxypyridine

| Conditions | Yield |
|---|---|
| Stage #1: allyl alcohol With sodium at 80℃; for 3h; Stage #2: 3,5-dichloropyridine In dimethyl sulfoxide at 60℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: butan-1-ol With sodium at 80℃; for 3h; Stage #2: 3,5-dichloropyridine In dimethyl sulfoxide at 70℃; for 10h; | 100% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
766-77-8
Dimethylphenylsilane

-
-
1308789-48-1
3,5-dichloro-1-[dimethyl(phenyl)silyl]-1,4-dihydropyridine

| Conditions | Yield |
|---|---|
| With C29H38PRuS(1+)*C32H12BF24(1-) In neat (no solvent) at 20℃; for 12h; Inert atmosphere; Glovebox; | 98% |
| With tris(pentafluorophenyl)borate In (2)H8-toluene at 85℃; for 5h; Inert atmosphere; chemoselective reaction; | 92 %Spectr. |
-
-
2457-47-8
3,5-dichloropyridine

-
-
15177-57-8
3,5-dicholoropyridine N-oxide

| Conditions | Yield |
|---|---|
| With peracetic acid In chloroform; acetic acid at 50℃; for 67h; | 96% |
| With dihydrogen peroxide; methyltrioxorhenium(VII) In dichloromethane; water at 24℃; for 17h; | 94% |
| With bis-trimethylsilanyl peroxide; per-rhenic acid In dichloromethane; water at 24℃; for 20h; | 90% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
5188-07-8
sodium thiomethoxide

-
-
98627-01-1
5-Chloro-3-methylsulfenylpyridine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Heating; | 95% |
| In N,N-dimethyl-formamide at 20℃; for 1h; |
-
-
2457-47-8
3,5-dichloropyridine

-
-
98627-01-1
5-Chloro-3-methylsulfenylpyridine

| Conditions | Yield |
|---|---|
| With mesna In N,N-dimethyl-formamide Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; polyaniline-supported palladium In water at 100℃; for 6h; Suzuki coupling; | 95% |
| With caesium carbonate; dihydrogen dichloro-bis(di-tert-butylphosphinito-κP)palladium(2-) In 1,2-dimethoxyethane Suzuki-Miyaura cross-coupling; Heating; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine; benzaldehyde With tris(trimethylsilyl)amine; cesium fluoride In N,N-dimethyl-formamide at 20℃; for 6h; Stage #2: With methanol; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1.5h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine; pivalaldehyde With tris(trimethylsilyl)amine; cesium fluoride In N,N-dimethyl-formamide at 20℃; for 6h; Stage #2: With methanol; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1.5h; | 94% |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; XPhos; palladium diacetate In toluene; tert-butyl alcohol at 120℃; for 14h; Buchwald-Hartwig amination; | 93% |
-
-
67969-82-8
tetrafluoroboric acid diethyl ether

-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; | 93% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
112275-50-0
tert-butyl 1,4-diazepine-1-carboxylate

-
-
303161-70-8
4-(5-chloropyridin-3-yl)-[1,4]diazepane-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium t-butanolate; XPhos In toluene; tert-butyl alcohol at 120℃; for 18h; Buchwald-Hartwig reaction; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 90℃; for 2h; ethoxylation; | 91% |
| 89% | |
| In dimethyl sulfoxide at 80℃; for 1h; |
-
-
100-48-1
pyridine-4-carbonitrile

-
-
2457-47-8
3,5-dichloropyridine

-
-
847446-18-8
[4-(3,5-dichloro)pyridyl](4-pyridyl)ketimine

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 2h; | 91% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
136590-83-5
3,5-dichloropyridine-4-carboxaldehyde

-
-
847446-20-2
bis[4-(3,5-dichloro)pyridyl]methanol

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 2h; | 91% |
-
-
107-31-3
Methyl formate

-
-
2457-47-8
3,5-dichloropyridine

-
-
136590-83-5
3,5-dichloropyridine-4-carboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.5h; Stage #2: Methyl formate In tetrahydrofuran at -78℃; for 1.4h; Further stages.; | 90% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
75-07-0
acetaldehyde

-
-
1254473-66-9
1-(3,5-dichloropyridin-4-yl)ethan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine With n-butyllithium; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; hexanes at -78℃; Stage #2: acetaldehyde In tetrahydrofuran; hexanes at -78℃; for 3h; Stage #3: With water; ammonium chloride In tetrahydrofuran; hexanes at -78℃; | 90% |
| Stage #1: 3,5-dichloropyridine; acetaldehyde With n-butyllithium; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; hexane at -78℃; for 3h; Stage #2: With ammonium chloride In tetrahydrofuran; hexane; water at -78 - 20℃; | 90% |
| With n-butyllithium; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; hexane at -78℃; | 90% |
| Stage #1: 3,5-dichloropyridine With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 1h; Inert atmosphere; Stage #2: acetaldehyde In tetrahydrofuran at 20℃; for 2h; | 66% |
| With n-butyllithium; diisopropylamine In tetrahydrofuran at -78℃; for 3h; | 65% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 90% |

-
-
2457-47-8
3,5-dichloropyridine

-
-
195062-57-8
p-tolylboronic pinacol ester

-
-
26409-32-5
3,5-bis(4-methylphenyl)pyridine

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate; potassium hydroxide In neat (no solvent) at 110℃; for 24h; Suzuki-Miyaura Coupling; Schlenk technique; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With C14H22N2P(1+)*CF3O3S(1-) In [D3]acetonitrile at 28℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 89% |
-
-
2457-47-8
3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine With Bu3MgLi In tetrahydrofuran at -10℃; for 2h; Stage #2: With iodine In tetrahydrofuran at 20℃; for 18h; Further stages.; | 88% |
| Stage #1: 3,5-dichloropyridine With lithium diisopropyl amide In tetrahydrofuran at -40℃; for 1h; Inert atmosphere; Stage #2: With iodine In tetrahydrofuran at -78 - 20℃; for 1h; Inert atmosphere; | 44% |
| Multi-step reaction with 2 steps 1.1: Bu3MgLi / tetrahydrofuran / 2 h / -10 °C 1.2: D2O / tetrahydrofuran 2.1: I2 / tetrahydrofuran; H2O View Scheme |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; | 87% |
| In dimethyl sulfoxide at 70℃; for 2h; | 77% |
| In dimethyl sulfoxide at 80℃; for 1h; | 62% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
20607-43-6
sodium isopropanethiolate

-
-
98627-07-7
3,5-bis(isopropylthio)pyridine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 80℃; | 87% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
13473-01-3
5-chloro-2-methoxypyridine

| Conditions | Yield |
|---|---|
| With sodium methylate In N,N-dimethyl-formamide at 80℃; for 2h; | 87% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
98627-07-7
3,5-bis(isopropylthio)pyridine

| Conditions | Yield |
|---|---|
| With sodium isopropanethiolate In N,N-dimethyl-formamide at 80℃; for 15h; | 87% |
-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
2457-47-8
3,5-dichloropyridine

-
-
26409-33-6
3,5-bis-(4-methoxyphenyl)-pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-methoxy-benzene With magnesium In tetrahydrofuran Metallation; Stage #2: 3,5-dichloropyridine With dibromobis(triphenylphosphine)nickel(II) In tetrahydrofuran Grignard reaction; Further stages.; | 86% |
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); magnesium; ethylene dibromide In tetrahydrofuran at 0℃; Inert atmosphere; Reflux; | 73% |
-
-
2457-47-8
3,5-dichloropyridine

-
-
343781-45-3
4-bromo-3,5-dichloropyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dichloropyridine With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -75℃; for 2h; Stage #2: With bromine In tetrahydrofuran; hexane at -75℃; for 3h; | 86% |
3,5-Dichloropyridine Chemical Properties
3,5-Dichloropyridine (2457-47-8) is also named as Dichloropyridine,98% ; 3,5-Dichloroypyridine ; 3,5-Dichloropyridine,97% ; Pyridine, 3,5-dichloro- ; WLN: T6NJ CG EG ; CCRIS 1719 ; D73804_ALDRICH ; NSC60546 ; EINECS 219-537-9 ,and so on.
3,5-Dichloropyridine (2457-47-8) is usually white to yellow powder.
IUPAC Name: 3,5-dichloropyridine
CAS: 2457-47-8
Molecular Formula: C5H3Cl2N
Molecular Weight: 147.99
Molecular structure: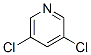
EINECS: 219-537-9
Melting point: 65-67°C
BRN: 1973
ACD/LogD (pH 5.5): 2.43
ACD/LogD (pH 7.4): 2.43
ACD/BCF (pH 5.5): 41.03
ACD/BCF (pH 7.4): 41.03
ACD/KOC (pH 5.5): 496.91
ACD/KOC (pH 7.4): 496.92
H bond acceptors: 1
H bond donors: 0
Freely Rotating Bonds: 0
Index of Refraction: 1.553
Molar Refractivity: 34.13 cm3
Molar Volume: 106.5 cm3
Polarizability: 13.53 10-24cm3
Surface Tension: 43.1 dyne/cm
Density: 1.388 g/cm3
Flash Point: 73.4 °C
Enthalpy of Vaporization: 39.32 kJ/mol
Boiling Point: 173.7 °C at 760 mmHg
Vapour Pressure: 1.67 mmHg at 25°C
3,5-Dichloropyridine Uses
3,5-Dichloropyridine (2457-47-8) is commonly used as pharmaceutical intermediate and pesticide intermediate.
3,5-Dichloropyridine Toxicity Data With Reference
RTECS: US8575000
| mouse | LD50 | intraperitoneal | 1180mg/kg (1180mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ANTIPSYCHOTIC LIVER: FATTY LIVER DEGERATION | Toxicology and Applied Pharmacology. Vol. 11, Pg. 361, 1967. |
3,5-Dichloropyridine Safety Profile
Hazard Codes: Xn,Xi
Risk Statements: 22-37/38-41-36/37/38 (22: Harmful if swallowed 37/38: Irritating to respiratory system and skin 41: Risk of serious damage to eyes 36/37/38: Irritating to eyes, respiratory system and skin)
Safety Statements: 26-39-36 (26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice 39: Wear eye/face protection 36: Wear suitable protective clothing)
Hazard Note: Irritant
3,5-Dichloropyridine Specification
Removal in wastewater treatment of 3,5-Dichloropyridine (2457-47-8) can be stated as follows:
Total removal:3.51 percent
Total biodegradation:0.10 percent
Total sludge adsorption:3.17 percent
Total to Air:0.24 percent
(using 10000 hr Bio P,A,S)
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 2457-48-9
- 245759-64-2
- 245762-35-0
- 245765-71-3
- 24577-34-2
- 2457-76-3
- 24577-68-2
- 2457-80-9
- 24579-73-5
- 24579-90-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View