-
Name
THIOBENZAMIDE
- EINECS 218-765-6
- CAS No. 2227-79-4
- Article Data187
- CAS DataBase
- Density 1.182 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 113-117 °C(lit.)
- Formula C7H7NS
- Boiling Point 245 °C at 760 mmHg
- Molecular Weight 137.205
- Flash Point 102 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance Yellowish-green crystalline powder
- Safety 45
- Risk Codes 25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Benzamide,thio- (6CI,7CI,8CI);Aminothiocarbonylbenzene;Benzenethiocarboxamide;Benzothioamide;Thiobenzamide;
- PSA 58.11000
- LogP 2.02110
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine; diammonium sulfide; triethylamine In water at 50℃; | 100% |
| With sodium hydrogensulfide; diethyl amine hydrochloride In 1,4-dioxane; water at 55℃; for 6h; | 98% |
| With diisopropyldithiophosphoric acid In methanol at 60℃; for 4h; | 97% |

| Conditions | Yield |
|---|---|
| With alumina-supported P2S5 In tetrahydrofuran at 60℃; for 0.0833333h; Microwave irradiation; | 98% |
| With 2,4-{[3-[(CH2)5C8F17]-4-MeO-phenyl]}2-P2S2 2,4-disulfide In tetrahydrofuran for 1.5h; | 97% |
| With 2,4-bis(4-(5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-heptadecafluorododecyloxy)phenyl)-1,3,2,4-dithiadiphosphetane 2,4-disulfide In tetrahydrofuran at 55℃; for 4h; | 94% |
-
-
7047-10-1
5-phenyl-3H-1,2,4-dithiazole-3-one

-
A
-
7783-06-4
hydrogen sulfide

-
B
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With carbonic anhydrase from bovine erythrocytes; GLUTATHIONE In aq. phosphate buffer; dimethyl sulfoxide at 20℃; for 4h; pH=7.4; Reagent/catalyst; Enzymatic reaction; | A n/a B 98% |
-
-
463962-26-7
thiobenzoyl (5,5-dimethyl-2-thioxo-1,3,2-dioxaphosphinan-2-yl) sulfide

-
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With pyridine; ammonium hydroxide In benzene Thioacylation; | 95% |
| With ammonium hydroxide In benzene | 94.5% |
-
-
110-91-8
morpholine

-
-
141-84-4
2-thioxo-4-thiazolidinone

-
-
55-21-0
benzamide

-
A
-
16781-67-2
2-morpholin-4-yl-2-thiazole-4(5H)-one

-
B
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With MCM-41 mesoporous silica In ethanol; water for 8h; Reagent/catalyst; Solvent; Temperature; Time; Reflux; Green chemistry; | A n/a B 89% |


| Conditions | Yield |
|---|---|
| With ammonium sulfide; trichlorotitanium(IV) trifluoromethanesulfonate; 1-n-butyl-3-methylimidazolim bromide at 80℃; for 1h; Ionic liquid; chemoselective reaction; | 87% |
| Multi-step reaction with 3 steps 1: trichlorothiophosphine; water; triethylamine / 70 - 75 °C / Neat (no solvent) 2: trichlorothiophosphine; water; triethylamine / 80 - 85 °C / Neat (no solvent) 3: water / 2.5 h / 80 - 85 °C / Neat (no solvent) View Scheme |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate; O,O-Diethyl hydrogen phosphorodithioate In 1,4-dioxane at 80 - 90℃; for 3h; Inert atmosphere; | 85% |
| Stage #1: benzaldehyde With ammonia; iodine In water at 20℃; for 0.5h; Green chemistry; Stage #2: With O,O-Diethyl hydrogen phosphorodithioate In water at 90℃; for 2h; Solvent; Temperature; Time; Green chemistry; | 85% |
| With sulfur; ammonium acetate In N,N-dimethyl-formamide at 100℃; for 5h; Green chemistry; | 83% |
-
-
20605-40-7
thiobenzoylhydrazine

-
A
-
96134-33-7
N,N-diethyl-5-phenyl-1,3,4-thiadiazol-2-amine

-
B
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 1h; Ambient temperature; | A 78% B 84% |


| Conditions | Yield |
|---|---|
| In acetonitrile Ambient temperature; | A 83% B 53% |

-
-
100-47-0
benzonitrile

-
-
298-06-6
O,O-Diethyl hydrogen phosphorodithioate

-
A
-
68206-76-8
N-(O,O-diethylthiophosphoryl)thiobenzamide

-
B
-
71039-20-8
O,O-diethyl-N-thiobenzoylamidophosphate

-
C
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| In water at 80℃; for 1.5h; | A 4.2% B 4.1% C 82.5% |


| Conditions | Yield |
|---|---|
| With sulfur; formamide; sodium hydroxide at 100℃; for 8h; Schlenk technique; | 79% |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
100-51-6
benzyl alcohol

-
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With caesium bromide; salicylaldehyde; sulfur; potassium hydroxide at 20℃; for 12h; Inert atmosphere; | 75.8% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water at 25℃; for 48h; regioselective reaction; | 74% |
-
-
101185-06-2
2-Cyan-3-phenyl-3-thiobenzoylamido-acrylsaeureethylester

-
A
-
3336-69-4
2-cyano-3-amino-3-phenyl acrylic acid ethyl ester

-
B
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With ammonium acetate In methanol for 0.0833333h; Heating; | A 71% B 48% |

| Conditions | Yield |
|---|---|
| With sulfur at 170℃; for 0.25h; Microwave irradiation; neat (no solvent); | A 71% B 2.4% |
-
-
80784-80-1
2-Phenyl-5,6-dihydro-1,3,5-thiadiazine-4,6-dione

-
A
-
100-47-0
benzonitrile

-
B
-
2227-79-4
benzenecarbothioamide

-
C
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 25℃; for 1h; Product distribution; other thiadiazinediones; var. pH; | A 8% B 14% C 65% |

-
-
21198-48-1
4,4-diethyl-thiosemicarbazide

-
A
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With triethylamine In water for 0.5h; Ambient temperature; | A 55% B 63% |

-
-
90889-28-4
O,O'-ethylene bis(hydrogen methylphosphonodithioate)

-
-
100-47-0
benzonitrile

-
A
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| In tetrachloromethane for 72h; Ambient temperature; | A 63% B 25% |

-
-
100-47-0
benzonitrile

-
-
106814-54-4
O,O'-trimethylene bis(hydrogen methylphosphonodithioate)

-
A
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| In tetrachloromethane Ambient temperature; | A 63% B 7% |


-
A
-
35547-63-8
5-dimethylamino-3-phenyl-1,2,4-thiadiazole

-
B
-
6972-05-0
1,1-dimethyl-2-thiourea

-
C
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In water for 12h; Ambient temperature; | A 1% B 62% C 27% |

-
-
68206-76-8
N-(O,O-diethylthiophosphoryl)thiobenzamide

-
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With water at 80℃; for 3h; | 60% |
-
-
100-47-0
benzonitrile

-
-
298-06-6
O,O-Diethyl hydrogen phosphorodithioate

-
A
-
68206-76-8
N-(O,O-diethylthiophosphoryl)thiobenzamide

-
B
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| at 80℃; for 2h; | A 58% B 16% |
| at 80℃; for 2h; | A 58% B 9.1% |

-
-
64-19-7
acetic acid

-
-
100-47-0
benzonitrile

-
-
107-56-2
O,O-diisopropyl hydrogen phosphorodithioate

-
A
-
3253-29-0
O,O,O,O-tetraisopropyl pyrophosphorotrithioate

-
B
-
24420-17-5
S-acetyl O,O-diisopropyl phosphorodithioate

-
C
-
66078-55-5
N-(diisopropoxythiophosphoryl)thiobenzamide

-
D
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| for 120h; Mechanism; Ambient temperature; other nucleophiles; | A n/a B n/a C n/a D 54% |

-
-
100-47-0
benzonitrile

-
-
107-56-2
O,O-diisopropyl hydrogen phosphorodithioate

-
A
-
3253-29-0
O,O,O,O-tetraisopropyl pyrophosphorotrithioate

-
B
-
24420-17-5
S-acetyl O,O-diisopropyl phosphorodithioate

-
C
-
66078-55-5
N-(diisopropoxythiophosphoryl)thiobenzamide

-
D
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With acetic acid for 120h; Ambient temperature; Yields of byproduct given; | A n/a B n/a C n/a D 54% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide Heating; | 53% |
-
-
105314-02-1
Thiobenzoyl-thiophosphoramidic acid O,O'-dimethyl ester

-
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With water at 80℃; for 3h; | 50% |
-
-
100-47-0
benzonitrile

-
-
107-56-2
O,O-diisopropyl hydrogen phosphorodithioate

-
A
-
66078-55-5
N-(diisopropoxythiophosphoryl)thiobenzamide

-
B
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| In diethyl ether for 456h; Ambient temperature; Yields of byproduct given; | A 46% B n/a |
| at 20℃; for 9h; | A 44% B 28% |


-
A
-
2227-79-4
benzenecarbothioamide

-
B
-
14478-73-0
3,6-diphenyl-1,4-dihydro-1,2,4,5-tetrazine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In water for 2h; Ambient temperature; | A 30% B 3% C 43% |


| Conditions | Yield |
|---|---|
| With triethylamine; trichlorophosphate In water at 65 - 75℃; for 0.1h; microwave irradiation; | A n/a B 43% |

| Conditions | Yield |
|---|---|
| With p-toluene sulfinic acid In water at 80℃; for 4.5h; | 100% |
| With 1,3-dibromo-5,5-diphenylimidazolidine-2,4-dione; water at 20℃; for 0.0833333h; neat (no solvent); | 99% |
| With tert-butylhypochlorite In tetrachloromethane for 0.0833333h; Ambient temperature; | 98% |
-
-
2227-79-4
benzenecarbothioamide

-
-
74-88-4
methyl iodide

-
-
40780-81-2
S-methyl thioimidate of benzoic acid

| Conditions | Yield |
|---|---|
| In acetone for 5h; Heating / reflux; | 100% |
| Ambient temperature; | 98% |
| In acetone Ambient temperature; |
-
-
21916-15-4
2-Chlormethylen-α-tetralon

-
-
2227-79-4
benzenecarbothioamide

-
-
83251-64-3
Thiobenzimidic acid 1-oxo-3,4-dihydro-1H-naphthalen-(2Z)-ylidenemethyl ester; compound with perchloric acid

| Conditions | Yield |
|---|---|
| With perchloric acid In acetic acid | 100% |
-
-
416860-02-1
N-[2-(4-bromoacetyl-phenyl)-ethyl]-acetamide

-
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| In ethanol | 100% |
| In ethanol at 80℃; for 3h; |
-
-
1062602-35-0
3-(phenylsulfanyl)-1-(thiophen-2-yl)prop-2-yn-1-ol

-
-
2227-79-4
benzenecarbothioamide

-
-
1167412-44-3
2-phenyl-5-(phenylsulfanyl)-4-(2-thienylmethyl)-thiazole

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; scandium tris(trifluoromethanesulfonate) In nitromethane; water for 0.166667h; Reflux; regioselective reaction; | 100% |
| With tetra(n-butyl)ammonium hydrogensulfate; scandium tris(trifluoromethanesulfonate) In nitromethane; water for 0.166667h; Reflux; |
-
-
1167412-36-3
1-(4-fluorophenyl)-3-(phenylsulfanyl)prop-2-yn-1-ol

-
-
2227-79-4
benzenecarbothioamide

-
-
1167412-41-0
4-(4-fluorobenzyl)-2-phenyl-5-(phenylsulfanyl)thiazole

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; scandium tris(trifluoromethanesulfonate) In nitromethane; water for 0.166667h; Reflux; regioselective reaction; | 100% |
| With tetra(n-butyl)ammonium hydrogensulfate; scandium tris(trifluoromethanesulfonate) In nitromethane; water for 0.166667h; Reflux; |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 100℃; for 8h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 65℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen; sodium hydroxide In tetrahydrofuran; water at 20℃; for 3h; | 99% |
| With p-methoxybenzenetellurinic acid anhydride In dichloromethane for 0.5h; Ambient temperature; | 95% |
| With bis(4-methoxyphenyl)telluride; tetrabutylammonium acetate In water; acetonitrile electrolysis; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap In acetonitrile | 99% |
| With pyridine |

| Conditions | Yield |
|---|---|
| In ethanol at 95℃; for 3h; | 99% |
| With acetic anhydride In toluene Heating; | 79% |
| In ethanol for 3h; Reflux; | 74% |
-
-
598-79-8
2-Chloroacrylic acid

-
-
2227-79-4
benzenecarbothioamide

-
-
116077-21-5
S-(2-Carboxy-2-chlorethyl)thiobenzamid-hydrochlorid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In benzonitrile for 14h; Ambient temperature; | 99% |
-
-
2227-79-4
benzenecarbothioamide

-
-
490039-00-4
methyl 3-(bromoacetyl)azulene-1-carboxylate

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In ethanol for 1h; Hantzsch reaction; Heating; | 99% |
-
-
67-56-1
methanol

-
-
2227-79-4
benzenecarbothioamide

-
-
845907-84-8
2-benzyloxycarbonylamino-3-(2-phenyl-thiazol-4-yl)-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| for 12h; Heating / reflux; | 99% |

-
-
1113-59-3
bromopyruvic acid

-
-
2227-79-4
benzenecarbothioamide

-
-
7113-10-2
2-phenyl-1,3-thiazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 2h; Heating / reflux; | 99% |
| In 1,4-dioxane at 110℃; for 2h; | 0.8 g |
-
-
1253754-43-6
1,1-bis(p-fluorophenyl)-3-(phenylsulfanyl)prop-2-yn-1-ol

-
-
2227-79-4
benzenecarbothioamide

-
-
1253754-71-0
4-[bis(p-fluorophenyl)methyl]-2-phenyl-5-(phenylsulfanyl)-1,3-thiazole

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; scandium tris(trifluoromethanesulfonate) In nitromethane; water for 0.166667h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With water; caesium carbonate for 0.166667h; Microwave irradiation; | 99% |
-
-
534-07-6
1,3-Dichloroacetone

-
-
2227-79-4
benzenecarbothioamide

-
-
4771-31-7
2-phenyl-4-chloromethylthiazole

| Conditions | Yield |
|---|---|
| In ethanol for 4h; Reflux; | 98% |
| In ethanol for 2h; Reflux; | 90% |
| Stage #1: 1,3-Dichloroacetone; benzenecarbothioamide In acetone Inert atmosphere; Reflux; Stage #2: With sulfuric acid for 0.5h; Inert atmosphere; | 85% |
-
-
55986-20-4
N-(p-tolylsulfonyl)dibenzylselenimide

-
-
2227-79-4
benzenecarbothioamide

-
A
-
1842-38-2
dibenzyl selenide

-
B
-
70-55-3
toluene-4-sulfonamide

-
C
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| In methanol for 0.5h; Mechanism; Ambient temperature; | A 98% B 98% C 81% |

-
-
2227-79-4
benzenecarbothioamide

-
-
19429-85-7
N,N-dimethylacetamide diethyl acetal

-
-
67229-59-8, 102254-71-7
N1,N1-dimethyl-N2-thiobenzoylacetamidine

| Conditions | Yield |
|---|---|
| 98% |
-
-
80-63-7
methyl 2-chloroacrylate

-
-
2227-79-4
benzenecarbothioamide

-
-
116077-23-7
S-<2-Chlor-2-(methoxycarbonyl)ethyl>thiobenzamid-hydrochlorid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane for 72h; Ambient temperature; | 98% |
-
-
2227-79-4
benzenecarbothioamide

-
-
33027-12-2
o-bromoacetylbenzophenone

| Conditions | Yield |
|---|---|
| In 1,4-dioxane Ambient temperature; | 98% |
-
-
2227-79-4
benzenecarbothioamide

-
-
2632-13-5
2-Bromo-4'-methoxyacetophenone

-
-
2362-68-7
4-(4′-methoxyphenyl)-2-phenylthiazole

| Conditions | Yield |
|---|---|
| In ethylene glycol at 20℃; for 0.0833333h; | 98% |
| In ethanol Hantzsch thiazole synthesis; Heating; | |
| at 20℃; for 0.0833333h; grinding; neat (no solvent); |
-
-
1611471-28-3
C11H17NO2S*ClH

-
-
2227-79-4
benzenecarbothioamide

-
-
1056261-39-2
5-(2-methylpropyl)-2-phenyl-3H-thieno[2,3-d]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| In neat (no solvent) Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 67℃; Inert atmosphere; | 98% |
-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
1074-12-0
phenylglyoxal hydrate

-
-
2227-79-4
benzenecarbothioamide

| Conditions | Yield |
|---|---|
| With SBA-15*AEPH2-HPA In water at 50℃; for 0.25h; Catalytic behavior; Solvent; Temperature; Time; Green chemistry; | 98% |
| With vitamin B1 stabilized on Fe3O4 magnetic nanoparticles In water at 80℃; for 0.583333h; Catalytic behavior; Reagent/catalyst; Temperature; Solvent; Time; Green chemistry; | 93% |


| Conditions | Yield |
|---|---|
| With SBA-15*AEPH2-HPA In water at 50℃; for 0.25h; Green chemistry; | 98% |
| With vitamin B1 stabilized on Fe3O4 magnetic nanoparticles In water at 80℃; for 0.666667h; Green chemistry; | 84% |

-
-
5344-90-1
2-Aminobenzyl alcohol

-
-
2227-79-4
benzenecarbothioamide

-
-
224300-14-5
2-phenyl-4H-benzo[d][1,3]thiazine

| Conditions | Yield |
|---|---|
| With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide In ethyl acetate at 25℃; for 0.333333h; | 98% |
BENZENECARBOTHIOAMIDE Chemical Properties
Molecular Structure of BENZENECARBOTHIOAMIDE (2227-79-4):
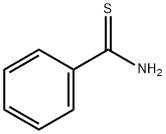
IUPAC Name: benzenecarbothioamide
Molecular Formula: C7H7NS
Molecular Weight: 137.20218 g/mol
XLogP3: 1.5
H-Bond Donor: 1
H-Bond Acceptor: 0
Canonical SMILES: C1=CC=C(C=C1)C(=S)N
InChI: InChI=1S/C7H7NS/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H2,8,9)
InChIKey: QIOZLISABUUKJY-UHFFFAOYSA-N
Index of Refraction: 1.652
Molar Refractivity: 42.44 cm3
Molar Volume: 116 cm3
Surface Tension: 60.2 dyne/cm
Density: 1.182 g/cm3
Flash Point: 102 °C
Boiling Point: 245 °C at 760 mmHg
Melting Point: 113-117 °C(lit.)
Enthalpy of Vaporization: 48.21 kJ/mol
Vapour Pressure: 0.0294 mmHg at 25 °C
Water Solubility: 4.678e+004 mg/L at 25 °C
BRN: 606021
BENZENECARBOTHIOAMIDE Toxicity Data With Reference
| 1. | mnt-mus-orl 180 µmol/kg | MUREAV Mutation Research, 192 (1987),141. | ||
| 2. | orl-mus LD50:95 mg/kg | THERAP Therapie, 8 (1953),237. | ||
| 3. | ipr-mus LD50:500 mg/kg | PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN, 11 (1977),1383. |
BENZENECARBOTHIOAMIDE Consensus Reports
Reported in EPA TSCA Inventory.
BENZENECARBOTHIOAMIDE Safety Profile
The safety information of BENZENECARBOTHIOAMIDE (2227-79-4):
Hazard Codes: T ![]()
Risk Statements: 25
25: Toxic if swallowed
Safety Statements: 45
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
RIDADR: UN 2811 6.1/PG 3
WGK Germany: 3
RTECS: CV5860000
HazardClass: 6.1
PackingGroup: III
Poison by ingestion. Moderately toxic by intraperitoneal route. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and SOx.
BENZENECARBOTHIOAMIDE Specification
BENZENECARBOTHIOAMIDE (2227-79-4) is yellowish-green crystalline powder with some other names such as Benzamide, thio- ; Benzothiamide ; Benzothioamide ; Phenylthioamide ; thio-benzamid ; Tiobenzamide ; THIOBENZAMIDE ; AKOS BBS-00004779 ,etc. According to its characteristics of molecular structural, it belongs to many categories such as phenyls;organic building blocks;sulfur compounds;thiocarbonyl compounds. Make sure store this product in a tightly closed container in a cool, dry place.
Related Products
- BENZENECARBOTHIOAMIDE
- Benzenecarbothioamide,2,4-dichloro-
- Benzenecarbothioamide,2,5-dimethoxy-
- Benzenecarbothioamide,2-bromo-
- Benzenecarbothioamide,2-hydroxy-
- Benzenecarbothioamide,2-methoxy-
- Benzenecarbothioamide,2-methyl-
- Benzenecarbothioamide,3,4-dichloro-
- Benzenecarbothioamide,3,5-dichloro-
- Benzenecarbothioamide,3,5-dimethoxy-
- 22278-81-5
- 22279-59-0
- 2227-98-7
- 22280-02-0
- 22280-56-4
- 22280-60-0
- 22280-62-2
- 22281-18-1
- 22282-58-2
- 22282-60-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View