-
Name
Imidazole
- EINECS 206-019-2
- CAS No. 288-32-4
- Article Data211
- CAS DataBase
- Density 1.116 g/cm3
- Solubility H2O: 0.1 M at 20 °C, clear, colorless
- Melting Point 88-91 °C(lit.)
- Formula C3H4N2
- Boiling Point 257 °C at 760 mmHg
- Molecular Weight 68.0782
- Flash Point 145 °C
- Transport Information UN 2923 8/PG 3
- Appearance white to off white crystals
- Safety 26-36/37/39-45-22-36-27
- Risk Codes 36/38-63-34-22-20/21/22
-
Molecular Structure
-
Hazard Symbols
 C,
C, Xi
Xi
- Synonyms Methanimidamide, N,N-1,2-ethenediyl-;1H-Imidazole (9CI);1H-Imidazole, monohydrochloride;1,3-Diazole;1,3-Diaza-2,4-cyclopentadiene;Imidazol;Methanimidamide, N,N-1, 2-ethenediyl-;Formamidine, N,N-vinylene-;Him;IMD;Glyoxaline;1467-16-9;Glyoxalin;Iminazole;Imutex;imidazole chloride;Pyrro(b)monazole;Miazole;2-phenyl-4,5-dihydroxy metylimidazole;
- PSA 28.68000
- LogP 0.40970
Synthetic route
-
-
18156-74-6
1-(Trimethylsilyl)imidazole

-
-
103-79-7
1-phenyl-acetone

-
A
-
288-32-4
1H-imidazole

-
B
-
43108-63-0
1-phenyl-2-trimethylsiloxy-1-propene

| Conditions | Yield |
|---|---|
| Ambient temperature; other silylazoles and carbonyl compounds; | A n/a B 100% |

-
-
49761-82-2
tert-butyl 1H-imidazole-1-carboxylate

-
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| With water at 100℃; for 0.166667h; | 99% |
| With silica gel In dichloromethane for 0.0333333h; Irradiation; | 98% |
| With water at 150℃; for 2h; Subcritical conditions; | 95% |
-
-
530-62-1
1,1'-carbonyldiimidazole

-
-
100-51-6
benzyl alcohol

-
A
-
288-32-4
1H-imidazole

-
B
-
22129-07-3
N-benzyloxycarbonylimidazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; for 12h; Esterification; | A n/a B 97% |


| Conditions | Yield |
|---|---|
| With Na2K-SG(I) In 1,2-dimethoxyethane at 20℃; Inert atmosphere; | 96% |
| With triethylsilane; palladium 10% on activated carbon In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 92% |
-
-
50-00-0
formaldehyd

-
-
131543-46-9
Glyoxal

-
-
7664-41-7
ammonia

-
-
141-43-5
ethanolamine

-
A
-
288-32-4
1H-imidazole

-
B
-
1615-14-1
1-(2-Hydroxyethyl)imidazole

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; ammonia; ethanolamine In water at 25℃; for 1h; Stage #2: Glyoxal; ammonia at 25℃; for 1h; Product distribution / selectivity; | A 2% B 96% |

-
-
64-17-5
ethanol

-
-
530-62-1
1,1'-carbonyldiimidazole

-
A
-
288-32-4
1H-imidazole

-
B
-
19213-72-0
ethyl 1-imidazolecarboxylate

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; for 12h; Esterification; | A n/a B 95% |
| at 20℃; |

-
-
131543-46-9
Glyoxal

-
-
78-84-2
isobutyraldehyde

-
A
-
288-32-4
1H-imidazole

-
B
-
36947-68-9
2-isopropylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: isobutyraldehyde With ammonia In methanol; water at 25℃; for 1h; Stage #2: Glyoxal With ammonia In methanol; water at 25℃; for 2h; Product distribution / selectivity; | A 2 %Chromat. B 95% |
| With ammonia In water at 25℃; for 1h; Product distribution / selectivity; | A 23 %Chromat. B 56% |

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
530-62-1
1,1'-carbonyldiimidazole

-
A
-
288-32-4
1H-imidazole

-
B
-
26654-39-7
4,4-dimethyl-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| In diethyl ether for 0.166667h; Ambient temperature; | A n/a B 94% |

-
-
24155-34-8
2-(1H-imidazol-1-yl)-1-phenylethanone

-
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| With magnesium; acetic acid In methanol at 20℃; | 93% |
-
-
50-00-0
formaldehyd

-
-
131543-46-9
Glyoxal

-
-
7664-41-7
ammonia

-
-
107-11-9
1-amino-2-propene

-
A
-
288-32-4
1H-imidazole

-
B
-
31410-01-2
1-allylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; ammonia; 1-amino-2-propene In water at 25℃; for 1h; Stage #2: Glyoxal; ammonia at 25℃; for 1h; | A 4% B 93% |


| Conditions | Yield |
|---|---|
| With lithium hydroxide; mercaptoacetic acid In N,N-dimethyl-formamide at 20℃; for 2h; | 92% |
| With mercaptoacetic acid; lithium hydroxide In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 92% |
| With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 24h; Inert atmosphere; Sealed tube; Irradiation; | 75% |
| With acetic anhydride In pyridine for 5h; Ambient temperature; |
-
-
2766-43-0
N-(tert-butoxycarbonyl)-L-serine methyl ester

-
-
530-62-1
1,1'-carbonyldiimidazole

-
A
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; for 12h; Esterification; | A n/a B 91% |

-
-
84661-56-3
1,1'-bis(imidazolyl)methane

-
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; acetic acid for 4.5h; | 91% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid at 25℃; for 0.0166667h; | 90% |
| With 3,3-dimethyldioxirane In methanol; acetone at 25℃; other imidazole derivatives; | 81% |
-
-
534-26-9
lysidine

-
A
-
288-32-4
1H-imidazole

-
B
-
1092546-18-3
2-imidazolinecarbonitrile

-
C
-
696650-10-9
2-imidazolinecarboxamide

| Conditions | Yield |
|---|---|
| With ammonia; MoO3-Sb2O4-TiO2 at 330℃; | A n/a B n/a C 90% |

-
-
15469-97-3
N-tritylimidazole

-
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| With Na2K-SG(I) In tetrahydrofuran at 20℃; Inert atmosphere; | 90% |
-
-
131543-46-9
Glyoxal

-
-
7664-41-7
ammonia

-
-
141-43-5
ethanolamine

-
-
75-07-0
acetaldehyde

-
A
-
288-32-4
1H-imidazole

-
B
-
1615-15-2
N-(2'-hydroxyethyl)-2-methylimidazole

| Conditions | Yield |
|---|---|
| Stage #1: ammonia; ethanolamine; acetaldehyde In water at 25℃; for 1h; Stage #2: Glyoxal; ammonia at 25℃; for 1h; | A 5% B 89% |

-
-
90-02-8
salicylaldehyde

-
-
73818-41-4
2-triphenyl(α-carboxymethylene)phosphorane imidazolide

-
A
-
288-32-4
1H-imidazole

-
B
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| Stage #1: salicylaldehyde With sodium methylate In 5,5-dimethyl-1,3-cyclohexadiene at 60℃; for 2h; Inert atmosphere; Stage #2: 2-triphenyl(α-carboxymethylene)phosphorane imidazolide In 5,5-dimethyl-1,3-cyclohexadiene for 48h; Intramolecular Wittig reaction; Reflux; Inert atmosphere; | A n/a B 85% |

-
-
31410-01-2
1-allylimidazole

-
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); diisobutylaluminium hydride In toluene for 1h; Ambient temperature; | 81% |
| With Na2K-SG(I) In tetrahydrofuran at 20℃; Inert atmosphere; | 65% |
-
-
110-85-0
piperazine

-
A
-
288-32-4
1H-imidazole

-
B
-
290-37-9
1,4-pyrazine

-
C
-
616-47-7
1-methyl-1H-imidazole

-
D
-
109-08-0
2-Methylpyrazine

-
E
-
13925-00-3
2-ethylpyrazine

-
F
-
693-98-1
2-methylimidazole

| Conditions | Yield |
|---|---|
| Pt-Al2O3-In2O3-Re at 400℃; Product distribution; variation of catalyst, temperature; | A n/a B 78.7% C n/a D 1.6% E 3.5% F n/a |


| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid at 106℃; for 0.0583333h; Microwave irradiation; | 78% |
-
-
34143-74-3
1H,1H,2H,2H-Perfluorodecanethiol

-
A
-
288-32-4
1H-imidazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 50℃; | A 76% B n/a |

-
-
574-19-6
1-(2-hydroxy-1-naphthyl)ethan-1-one

-
-
73818-41-4
2-triphenyl(α-carboxymethylene)phosphorane imidazolide

-
A
-
288-32-4
1H-imidazole

-
B
-
21568-13-8
1-methyl-3H-naphtho[2,1-b]pyran-3-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-(2-hydroxy-1-naphthyl)ethan-1-one With sodium methylate In 5,5-dimethyl-1,3-cyclohexadiene at 60℃; for 2h; Inert atmosphere; Stage #2: 2-triphenyl(α-carboxymethylene)phosphorane imidazolide In 5,5-dimethyl-1,3-cyclohexadiene for 48h; Intramolecular Wittig reaction; Reflux; Inert atmosphere; | A n/a B 75% |

-
-
118-93-4
o-hydroxyacetophenone

-
-
73818-41-4
2-triphenyl(α-carboxymethylene)phosphorane imidazolide

-
A
-
288-32-4
1H-imidazole

-
B
-
607-71-6
4-methyl-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| Stage #1: o-hydroxyacetophenone With sodium methylate In 5,5-dimethyl-1,3-cyclohexadiene at 60℃; for 2h; Inert atmosphere; Stage #2: 2-triphenyl(α-carboxymethylene)phosphorane imidazolide In 5,5-dimethyl-1,3-cyclohexadiene for 48h; Intramolecular Wittig reaction; Reflux; Inert atmosphere; | A n/a B 75% |


| Conditions | Yield |
|---|---|
| With Na2K-SG(I) In tetrahydrofuran at 20℃; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide for 13h; Ambient temperature; | 100% |
| With sodium hydroxide; phase transfer catalysis by tetraethylammonium bromide for 16h; Ambient temperature; | 96% |
| With tetrabutylammomium bromide; sodium hydroxide Inert atmosphere; Reflux; | 87% |

| Conditions | Yield |
|---|---|
| In benzene at 8 - 20℃; | 100% |
| With iodine at 20℃; for 0.233333h; Neat (no solvent); | 97.32% |
| In benzene at 20℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; | 100% |
| In 1,4-dioxane at 0 - 20℃; | 87% |
| In diethyl ether for 3h; Heating; | 84% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; Inert atmosphere; | 100% |
| Stage #1: 1H-imidazole With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 1h; Stage #2: propyl bromide In tetrahydrofuran; mineral oil at 20℃; for 16h; | 93% |
| Stage #1: 1H-imidazole With sodium hydride In tetrahydrofuran; hexane at 20℃; Stage #2: propyl bromide In tetrahydrofuran; hexane at 60 - 65℃; Reflux; | 86% |
-
-
288-32-4
1H-imidazole

-
-
623-25-6
p-Xylylene dichloride

-
-
56643-83-5
1,4-bis(imidazol-l-yl-methyl)benzene

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 6h; | 100% |
| Stage #1: 1H-imidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 2h; Stage #2: p-Xylylene dichloride In N,N-dimethyl-formamide; mineral oil at 20℃; for 4h; | 100% |
| With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With iodine; sodium carbonate In 1,4-dioxane; water at 20℃; for 24h; | 100% |
| With dihydrogen peroxide; iodine In water at 50℃; for 24h; Green chemistry; | 97% |
| With pyridine; iodine; bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane for 3h; Ambient temperature; | 95% |
-
-
288-32-4
1H-imidazole

-
-
52379-63-2
2,4-dichloro-6-ethyl-5-nitropyrimidine

-
-
156489-94-0
6-ethyl-2,4-bis(1H-imidazol-1-yl)-5-nitropyrimidine

| Conditions | Yield |
|---|---|
| In acetonitrile for 18h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile for 2h; Ambient temperature; | 100% |
| Stage #1: 1H-imidazole With o-phenylenebis(diphenylphosphine); potassium tert-butylate; copper(l) chloride In toluene for 0.166667h; Michael Addition; Inert atmosphere; Stage #2: PVS In toluene at 22℃; for 3h; Michael Addition; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With 1,2,3-Benzotriazole; potassium tert-butylate; copper(l) iodide In dimethyl sulfoxide at 110℃; for 0.5h; | 100% |
| With potassium carbonate In dimethyl sulfoxide at 120℃; for 36h; | 97% |
| With potassium carbonate; trans-1,2-diaminocyclohexane-based copper(II) complex In N,N-dimethyl-formamide at 110℃; for 1.5h; | 92% |
-
-
288-32-4
1H-imidazole

-
-
76513-69-4
(2-trimethylethylsilylethoxy)methyl chloride

-
-
101226-33-9
1-((2-(trimethylsilyl)ethoxy)methyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole With sodium hydride In tetrahydrofuran at 0 - 25℃; for 0.5h; Inert atmosphere; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran at 0 - 25℃; for 14h; Inert atmosphere; | 100% |
| Stage #1: 1H-imidazole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.75h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In tetrahydrofuran; mineral oil at 20℃; | 93% |
| Stage #1: 1H-imidazole With potassium tert-butylate In dimethyl sulfoxide at 0℃; for 0.5h; Large scale; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In dimethyl sulfoxide at 0 - 15℃; for 16h; Large scale; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 2h; Stage #2: benzyl bromide In N,N-dimethyl-formamide at 20℃; for 4h; | 100% |
| Stage #1: 1H-imidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.25h; Stage #2: benzyl bromide In N,N-dimethyl-formamide; mineral oil at 25℃; for 3h; | 97% |
| With caesium carbonate at 100℃; for 0.416667h; Microwave irradiation; | 90% |
-
-
288-32-4
1H-imidazole

-
-
84446-19-5
1-oxo-11-(2'-chloroacetyl)-5,11-dihydro-6H-pyrido<2,3-b><1,4>benzodiazepin-6-one

-
-
84446-11-7
1-oxo-5,11-dihydro-11-imidazol-1'-ylacetyl-6H-pyrido<2,3-b><1,4>benzodiazepin-6-one

| Conditions | Yield |
|---|---|
| In toluene at 80℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With 1,2,3-Benzotriazole; potassium tert-butylate; copper(l) iodide In dimethyl sulfoxide at 110℃; for 0.5h; | 100% |
| With pyridine; potassium carbonate; copper(II) oxide at 115℃; Ullmann Condensation; | 96% |
| With potassium carbonate In acetonitrile for 24h; Heating; | 95% |
-
-
288-32-4
1H-imidazole

-
-
1129-28-8
3-methoxycarbonylbenzyl bromide

-
-
218131-31-8
methyl 3-((1H-imidazol-1-yl)methyl)benzoate

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 20h; | 100% |
| In N,N-dimethyl-formamide for 8h; | 67% |
| In acetone for 2h; Heating; | 55% |

| Conditions | Yield |
|---|---|
| H-Y zeolite at 299.9℃; | 100% |
| calcined Mg-Al layered double hydroxides at 424.85℃; Alkylation; | 63% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 48h; Ambient temperature; | 100% |

-
-
288-32-4
1H-imidazole

-
-
623-24-5
1,4-bis(bromomethyl)benzene

-
-
56643-83-5
1,4-bis(imidazol-l-yl-methyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 1H-imidazole With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 2h; Inert atmosphere; Stage #2: 1,4-bis(bromomethyl)benzene In tetrahydrofuran; mineral oil for 6h; Reflux; Inert atmosphere; | 100% |
| Stage #1: 1H-imidazole With potassium hydroxide In acetonitrile at 20℃; for 2h; Stage #2: 1,4-bis(bromomethyl)benzene In acetonitrile at 20℃; for 1.5h; | 88% |
| With potassium hydroxide In isopropyl alcohol | 80% |
-
-
288-32-4
1H-imidazole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
49761-82-2
tert-butyl 1H-imidazole-1-carboxylate

| Conditions | Yield |
|---|---|
| With 1-methylimidazolium tetrafluoroborate at 30 - 35℃; for 0.0833333h; | 100% |
| With guanidine hydrochloride In ethanol at 35 - 40℃; for 0.0166667h; | 100% |
| With amberlyst-15 In ethanol at 20℃; for 0.0166667h; chemoselective reaction; | 100% |
-
-
288-32-4
1H-imidazole

-
-
81903-80-2
3,4-dibromophenylsulfonic acid chloride

-
-
224824-30-0
1-((3,4-dibromophenyl)sulfonyl)-1H-imidazole

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 19h; | 100% |
| With sodium carbonate In methanol; chloroform for 22h; Ambient temperature; | 34% |
-
-
288-32-4
1H-imidazole

-
-
156441-21-3
(2R,4R)-1-allyloxycarbonyl-4-tert-butyldimethylsilyloxy-2-(2-methanesulfonyloxyethyl)pyrrolidine

-
-
156441-22-4
(2R,4R)-1-allyloxycarbonyl-4-tert-butyldimethylsilyloxy-2-[2-(imidazol-1-yl)ethyl]pyrrolidine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide at 60℃; for 1h; Alkylation; | 100% |

| Conditions | Yield |
|---|---|
| With [Cu4I4(1,4-diazabicyclo[2.2.2]octane)2]n In methanol at 27℃; for 5h; | 100% |
| With [Cu30I16(5-methyl-4-(p-tolyl)pyrimidine-2-thiolato)12(μ10-S4)] In methanol for 5h; Temperature; Reagent/catalyst; Reflux; | 99% |
| With polystrene supported copper furfural catalyst In methanol at 40℃; for 10h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In toluene for 5h; Heating; | 100% |
| In toluene at 20℃; | 91.9% |
| In toluene to soln. B(C6F5)3 and imidazole in toluene triethylamine in toluene wasadded, react. mixt. was stirred overnight at room temp.; soln. was concd., hexane was added, ppt. was filtered, washed with hexane and dried under reduced pressure; elem. anal.; | 91.9% |


| Conditions | Yield |
|---|---|
| potassium carbonate In N,N-dimethyl-formamide at 100℃; for 8h; | 100% |
| With tripotassium phosphate "n" hydrate In N,N-dimethyl-formamide at 110℃; for 18h; | 50% |
| With potassium carbonate; N,N-dimethyl-formamide at 100℃; for 48h; Inert atmosphere; | 46% |
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; | |
| potassium carbonate In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With copper phthalocyanine; sodium hydroxide In dimethyl sulfoxide at 100℃; | 100% |
| With Hippuric Acid; copper diacetate; caesium carbonate In N,N-dimethyl-formamide at 140℃; for 30h; Ullmann coupling reaction; | 99% |
| With caesium carbonate In dimethyl sulfoxide at 110℃; for 1h; Ullmann Condensation; | 98% |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; 1,10-Phenanthroline; tetrabutyl ammonium fluoride at 140 - 145℃; for 24h; | 100% |
| With potassium tert-butylate; trans-1,2-diaminocyclohexane-based copper(II) complex In N,N-dimethyl-formamide at 110℃; for 2h; | 99% |
| With copper(l) iodide; caesium carbonate In N,N-dimethyl-formamide at 20 - 100℃; for 24.5h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper(I) oxide In N,N-dimethyl-formamide at 80℃; for 24h; | 100% |
| With potassium tert-butylate In toluene at 180℃; for 18h; Ullmann condensation; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 6h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With 1-sulfanyl-2-(dimethylaminomethyl)-3-Me3Si-benzene-Cu(I); potassium carbonate In 1-methyl-pyrrolidin-2-one at 160℃; for 16h; | 100% |
| With copper(l) iodide; potassium carbonate In dimethyl sulfoxide at 150℃; for 48h; | 99% |
| With copper(l) iodide; dimethylaminoacetic acid; potassium carbonate In dimethyl sulfoxide at 110℃; for 48h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; 1,10-Phenanthroline; tetrabutyl ammonium fluoride at 140 - 145℃; for 24h; | 100% |
| With copper(l) iodide; caesium carbonate In N,N-dimethyl-formamide at 20 - 100℃; for 24.5h; | 99% |
| With potassium carbonate; copper(l) iodide In N,N-dimethyl-formamide at 110℃; for 24h; Ullmann coupling reaction; | 98% |
-
-
871946-66-6
6-(bromoacetyl)-2-butyl-4-methyl-3,4-dihydro-2H-1,4-benzoxazin-3-one

-
-
288-32-4
1H-imidazole

-
-
871946-70-2
2-butyl-6-(1H-1-imidazolylacetyl)-4-methyl-3,4-dihydro-2H-1,4-benzoxazin-3-one

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 5h; | 100% |
Imidazole Chemical Properties
Structure of Imidazole (CAS NO.288-32-4):
![]()
Molecular Formula: C3H4N2
Molecular Weight: 68.08 g/mol
Index of Refraction: 1.528
Density: 1.116 g/cm3
Flash Point: 145 °C
Enthalpy of Vaporization: 47.46 kJ/mol
Boiling Point: 257 °C at 760 mmHg
Vapour Pressure: 0.024 mmHg at 25 °C
Melting point: 88-91 °C(lit.)
Storage tempreture: 2-8 °C
Solubility: H2O: 0.1 M at 20 °C, clear, colorless
Appearance: White to off white crystals
IUPAC Name: 1H-Imidazole
Product Category of Imidazole (CAS NO.288-32-4): Imidazoles, Pyrroles, Pyrazoles, Pyrrolidines ; Reagents for Oligosaccharide Synthesis ; Zone Refined Products ; Buffer
Imidazole History
In 1858, Imidazole (CAS NO.288-32-4) was first synthesized by Heinrich Debus. But various imidazole derivatives had been discovered as early as the 1840s.
Imidazole Production
The synthesis of Imidazole (CAS NO.288-32-4) is shown below, and you can use glyoxal and formaldehyde in ammonia to form imidazole. This synthesis, while producing relatively low yields, is still used for creating C-substituted imidazoles.
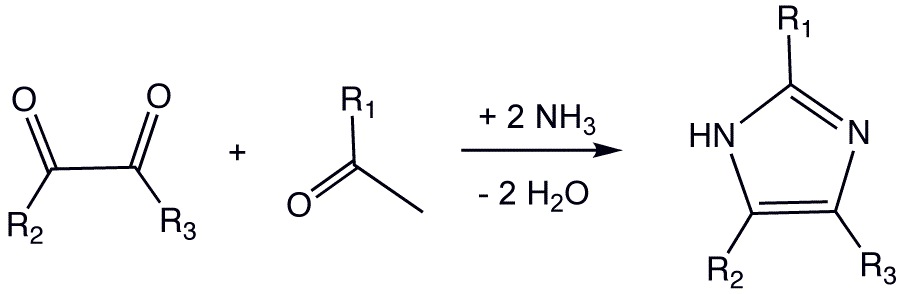
In one microwave modification the reactants are benzil, formaldehyde and ammonia in glacial acetic acid forming 2,4,5-triphenylimidazole (Lophine).
Imidazole Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | subcutaneous | 125mg/kg (125mg/kg) | BEHAVIORAL: FOOD INTAKE (ANIMAL) BEHAVIORAL: REGIDITY | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 84, Pg. 155, 1918. |
| dog | LD50 | subcutaneous | 28mg/kg (28mg/kg) | Przeglad Epidemiologiczny. Vol. 67, Pg. 295, 1993. | |
| guinea pig | LD50 | oral | 760mg/kg (760mg/kg) | Przeglad Epidemiologiczny. Vol. 67, Pg. 295, 1993. | |
| mammal (species unspecified) | LD50 | oral | 1gm/kg (1000mg/kg) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 608, 1966. | |
| mouse | LD50 | intraperitoneal | 300mg/kg (300mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | intravenous | 475mg/kg (475mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Arzneimittel-Forschung. Drug Research. Vol. 33, Pg. 716, 1983. |
| mouse | LD50 | oral | 880mg/kg (880mg/kg) | German Offenlegungsschrift Patent Document. Vol. #3046325, | |
| mouse | LD50 | subcutaneous | 817mg/kg (817mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 119, Pg. 444, 1957. | |
| rat | LD50 | oral | 220mg/kg (220mg/kg) | Przeglad Epidemiologiczny. Vol. 67, Pg. 295, 1993. | |
| rat | LD50 | subcutaneous | 626mg/kg (626mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 119, Pg. 444, 1957. |
Imidazole Safety Profile
Hazard Codes:  C,
C, Xi
Xi
Risk Statements: 36/38-63-34-22-20/21/22
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R22:Harmful if swallowed.
R34:Causes burns.
R36/38:Irritating to eyes and skin.
R63:Possible risk of harm to the unborn child.
Safety Statements: 26-36/37/39-45-22-36-27
S22:Do not breathe dust.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S27:Take off immediately all contaminated clothing.
S36:Wear suitable protective clothing.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
Imidazole Specification
Imidazole , its cas register number is 288-32-4. It also can be called 1,3-Diazole ; 1H-Imidazole ; and Glyoxaline . It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately. Notes to physician: Treat supportively and symptomatically.
In addition, Imidazole (CAS NO.288-32-4) could be stable under normal temperatures and pressures. It is not compatible with strong oxidizing agents, acids, acid chlorides, and you must not take it with incompatible materials, dust generation. And also prevent it to broken down into hazardous decomposition products: hydrogen cyanide, nitrogen oxides, carbon monoxide, carbon dioxide, ammonia.
Related Products
- Imidazole
- imidazole acetic acid
- Imidazole hydrochloride
- Imidazole mustard
- Imidazole trifluoromethanesulfonate
- Imidazole, 1-dodecyl-
- Imidazole, 5-methyl-1-phenyl-2-(pyrrolidinyl)-
- Imidazole, Sodium Derivative
- Imidazole-2-carboxaldehyde
- Imidazole-2-thiol
- 28833-81-0
- 2883-45-6
- 28836-03-5
- 288-36-8
- 288383-20-0
- 288385-88-6
- 288385-93-3
- 288386-04-9
- 288386-15-2
- 288-39-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View